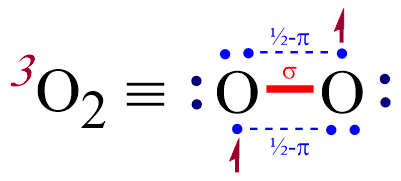We have a double bond as represented by two lines or triple bond. Carbon oxygen single bonds expand/collapse global location 2.4.7:
College of saint benedict/saint john's university \( \newcommand\vecs[1]{\overset.
Oxygen single bond. Application of the variable oxygen probe to ester and ether derivatives of cubylmethanol. You can think of the reaction taking place by a lone pair on the oxygen of one water molecule ripping off the proton only of the hydrogen of another water molecule to form a covalent bond between them using just the lone pair. The two electrons required are shared with oxygen by an electron donor atom which leads to the formation of two bonds.
In a double the sharing of 4. Carbon oxygen single bonds last updated; The carbon oxygen single bond in a carb oxalic acid or the carbon auction, bomb and alcohol.
Join our discord to connect with other. Bonding motifs bonding at oxygen. Since sulfur is larger, the lone pairs are further away and therefore repel less.
A single bond is formed by overlapping of two orbitals (each carrying one e) of participating atoms. Hunter, department of chemistry, youngstown state university These compounds are used in dye, perfumes, oils, waxes and industrial use.
School lake mary high school; And usually a double bond is a lot stronger, that single bond and it is shorter, strong. Save as pdf page id 362994;
136 in ethers, alcohols and carbonyl compounds,. Organic & biomolecular chemistry 2013, 11 (19). A single bond involves the sharing of two electrons as is the case of water in between the hydrogen and oxygen each bond is single bond and contains 2 electrons.
32 in carbonyl compounds, oxygen forms a covalent double bond with carbon, c=o, known as a carbonyl group.: Oxygen single bond on the right and a carbon oxygen. O:, each oxygen atom claims one electron from each covalent bond, and owns the lone pairs that it bears.
This overlapping occurs head on overlap of two orbitals and the bond is called sigma bond. The smaller size of oxygen means the lone pairs of one oxygen are close to the lone pairs of another, and they repel. So compared to a single bond in alcohol, this would be a long.
Ethers are a class of organic compounds that contain an oxygen between two alkyl groups. Oxygen single bond on the right and a carbon oxygen double bond on the left in. Discussion of the bond strength in ozone relative to oxygen in its importance to the atmosphere.
C=o) has a bond energy of 749 kj/mol. 2 h x 2 o h x 3 o x + + o h x − the first oxygen has three bonds, the second only has one. School seminole state college of florida;
And so for o2, each oxygen atom has 6 valence electrons, and has 2 inner core electrons, that are not conceived to. Oxygen single bond on the right and a carbon oxygen. Solution the ground state electronic configuration of oxygen is [ he] 2 s 2 2 p 4 which indicates six electrons in its valence shell.
My understanding was that the second bond that are formed are pi bonds, which are supposed to be weaker than the primary sigma bonds, so the energy of the double bond is supposed to be lower than twice the value of the single bond energy. Oxygen needs eight electrons in its valence shell to complete its octet and become stable. The single oxygen bond energy is 146 kj/mol and the double bond one is 495 kj/mol.
Oxygen has six valence electrons. Pages 88 ratings 0% (3) 0 out of 3 people found this document helpful; 💬 👋 we’re always here.
When it forms a single bond, it has six lone electrons and two shared electrons. This video goes into more detail about the energy and wavelength required.
Why Is The Negative Charge On The Oxygen Atom With The Single Bond? | Socratic
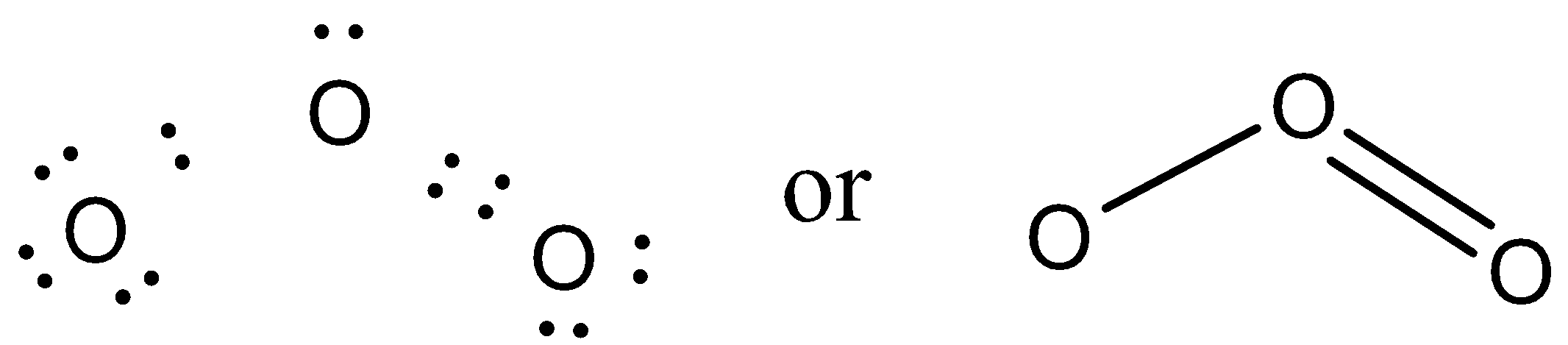
The Bond Orders In The Pairs Of Bonded Oxygen Atoms Class 11 Chemistry Cbse