For each compound, we can calculate the mass of 1 mole of that. Molar mass of iron iii oxide.

Oneclass: What Is The Molar Mass Of Iron (Iii) Acetate? (Please Show Any Work To Arrive At An Answer....
What is the molar mass of iron.
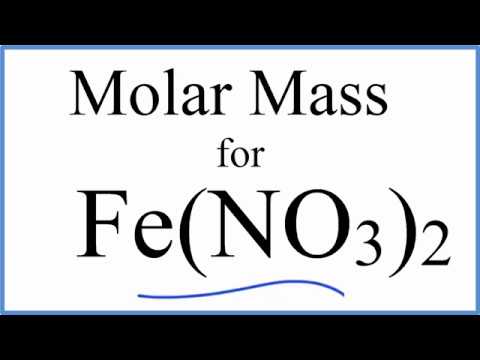
Molar amss of iron. Explanation of how to find the molar mass of fe2(so4)3: (1 atom x 56 grams/mole fe) + (2 atoms x 35.5 grams/mole of chlorine) = 127 grams/mole of iron (ii) chloride for other compounds, this might get a little bit. Molecular mass ( molecular weight) is the mass of one molecule of a substance and is expressed in the unified atomic mass units (u).
The molar mass of iron(iii) oxide is approximately 159.7 g/mole and is insoluble in water. Iron weighs 7.873 gram per cubic centimeter or 7 873 kilogram per cubic meter, i.e. Molar mass of fe2o3 (iron oxide) (our other math & science tools) molar weight of fe2o3 (iron oxide) formula = > weight theoretical yield percent yield uses the formula of a.
The units of molar mass follow its definition; Molar mass = the atomic mass of element × number of atoms. The molar mass will be equal to:
It is soluble in strong acids such as hydrochloric acid and sulfuric acid. Molar mass is basically atomic, molecular or formula mass of a substance expressed in grams instead of atomic mass unit (amu). Thus, for example, the molar.
For chemical elements without isolated molecules, such as carbon and metals, the molar mass is computed dividing by the number of moles of atoms instead. Since 69.9% and 30.1% of iron and oxygen are present in 100 grams of iron oxide. Density of iron is equal to 7 873 kg/m³;
What is the molar mass of iron (iii) nitrate (fe (no _3 3) _3 3 )? Molar mass of iron nitrate. The bridge between the particulate and the macroscopic levels is molar mass, the mass in grams of one mole of a substance.
Explanation of how to find the molar mass of feso4: The mass of 1 mole of a substance is called its molar mass. At 20°c (68°f or 293.15k) at standard atmospheric pressure.
As atomic mass of iron is approximately 56 amu (actually.
Atomic Weight Of Iron | Commission On Isotopic Abundances And Atomic Weights

1 Atom Of Iron (Fe) Weighs Grams.(Molecular Weight Of Fe = 56 G/Mole)