This unit will allow the student to discover the periodic trends and their relationship to atomic structure. Do not confuse these two sets of rules!step 3:
The reason for learning to draw lewis structures is to predict the number and type of.

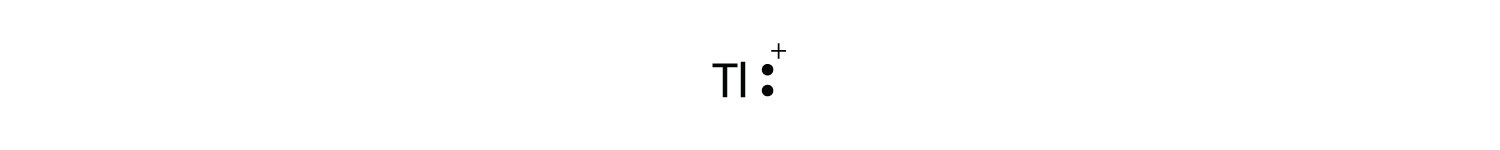
Lewis structure ti. Do this for each atom, one at a. Indicate the molecular shapes 3. The tetrahedral structure for ticl4 is consistent with its description as a d 0 metal center ( ti4+) surrounded by four identical ligands.
Chemical bonding is the study of the forces holding compounds together. This configuration leads to highly symmetrical structures,. Lewis structure molecular shape bond angles hybridization or formal charge for on central atom element in bold 16.) bf3 7.) nf3 *n 8.) h30* 9.) pf3 10.) [ch₂f2 *f draw the lewis structure.
Assembly and c programs must be. A fast, responsive, graphical periodic table program. Subtract the number of valence electrons (from step 1) from the number you got in step 2.
Generally, pure titanium can crystallize in two crystal structures: Understanding the periodic table is dependent upon learning the structure of the atom. A lewis structure is a graphic representation of the electron distribution around atoms.
By bonding with each other, atoms decrease in potential energy, and create more stable. Titanium atomic spectroscopy standard concentrate 1.00 g ti, 1.00 g/l, for 1 l standard solution, analytical standard 3 chemical and physical properties 3.1 computed properties 3.2. Α titanium and β titaniu.
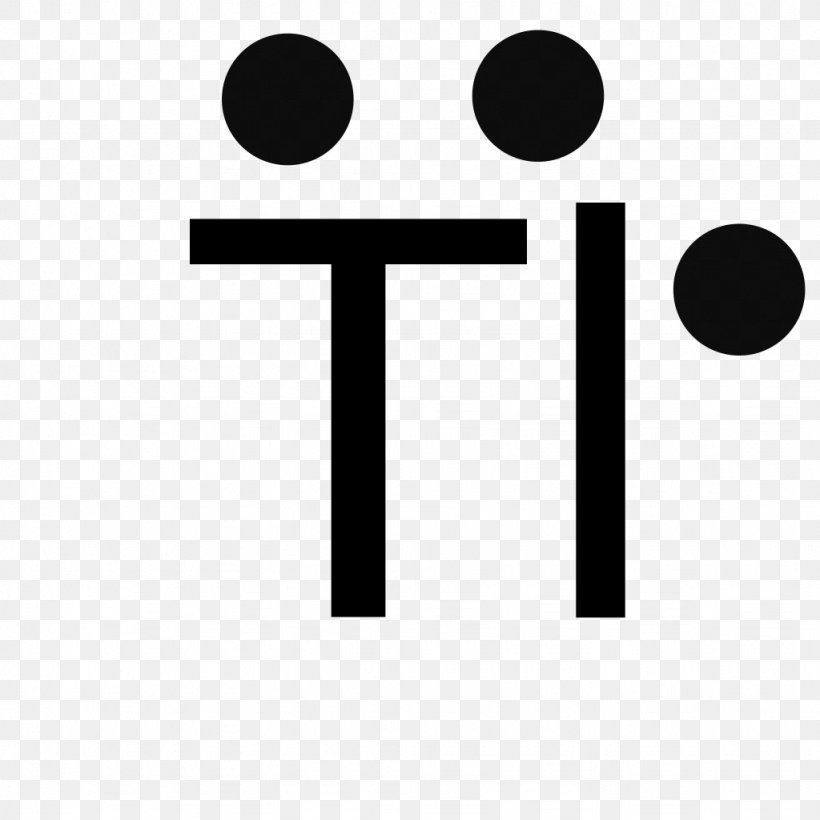
Lewis Structure Titanium Dioxide Diagram Electron, Png, 1024X1024Px, Lewis Structure, Area, Black And White, Bohr Model,