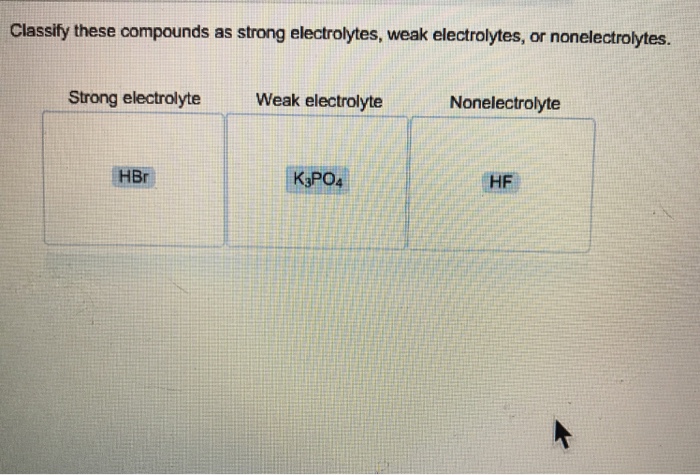
Hf, on the other hand will ionize in water (becoming h+ and f− ), but only to a small extent, because it is a weak acid. Reset help h and fatoms the completely dissociates into when it dissolves in water h' and fions when.
Solved Classify These Compounds As Strong Electrolytes, Weak | Chegg.com
The acetic acid is dissolved in the water and ionises as ethanoate and the hydronium ion.
Is hf a weak electrolyte. Expert answer 100% (6 ratings) transcribed image text: Therefore, the solution consists of few ions, and conducts very slightly. Hf, on the other hand will ionize in water (becoming h+ and f− ), but only to a small extent, because it is a weak acid.
The particular average football player sweats anywhere between 1. Why hf is a weak electrolyte? Fluoride acid is widely used as super acid in petrochemicals industries.
How is the solution of lif different from that of hf? Hf is the weak electrolyte. How is the solution of kf different from that of hf?
Now let’s discuss some examples of weak electrolytes: I have not attempted here to explain why hcl is strong while hf is weak. That is the reason, why hydrofluoric acid (hf) is a weak acid i.e.
Thus, this makes acetic acid a weak electrolyte. Match the words in the left column to the appropriate blanks in the sentences on the right. Therefore, it is also a strong electrolyte.
It is more difficult for the bond to be broken, so it will not completely dissociate in water. Match the words in the left column to the appropriate blanks in the sentences on the right. Therefore, the solution consists of few ions, and conducts very slightly.
Therefore, the solution consists of few ions, and conducts very slightly. Strong electrolytes and their examples the substances which ionize completely into ions are known as strong electrolytes. Hf is the weak electrolyte.
Hf is the weak electrolyte. Therefore, hf is a weak electrolyte. Part a lif is a strong electrolyte, and hf is a weak electrolyte.
It does not ionize completely when added to water. Reset help hf molecules the completely dissociates into when it dissolves in water. Even though hf is an acid, it is.
Kf is a strong electrolyte, and hf is a weak electrolyte. Examples of bases that are weak include ammonia and many more. Weak electrolytes include weak acids, weak bases, and a variety of other compounds.
Hf, on the other hand will ionize in water (becoming h + and f − ), but only to a small extent, because it is a weak acid. Therefore, it is also a strong electrolyte. Weak electrolytes only partially break into ions in water.
Fluoride acid this fluoride acid in gas or colorless fluid is commonly used as a dilute solution called hydrofluoric acid, but it is also a major source of fluorine industry. Hf is a weak acid because the bond between hydrogen and fluorine is so short. The extent of this loss will depend on the sort of physical activity, but more significantly the speed of sweat.
Most compounds that contain nitrogen are weak electrolytes. All athletes will experience some level of electrolyte loss and dehydration. Examples of bases that are strong electrolytes include sodium hydroxide and many more.
What makes something a strong or weak electrolyte? Another reason for the weakness of hf as acid is the presence of hydrogen bonding, but only if ‘hf’ is either pure or added to protic (h + containing) solvent having (f, o, or n) attached directly to the h atom. Is sugar a strong electrolyte?
Strong and weak electrolytes can also be applied to bases and other compounds that dissolve in water to produce ions. Examples of weak electrolytes include acetic acid (ch 3 cooh), carbonic acid (h 2 co 3 ), ammonia (nh 3 ), hydrogen fluoride (hf), hydrogen cyanide (hcn), and pyridine (c 2 h 5 n), etc.
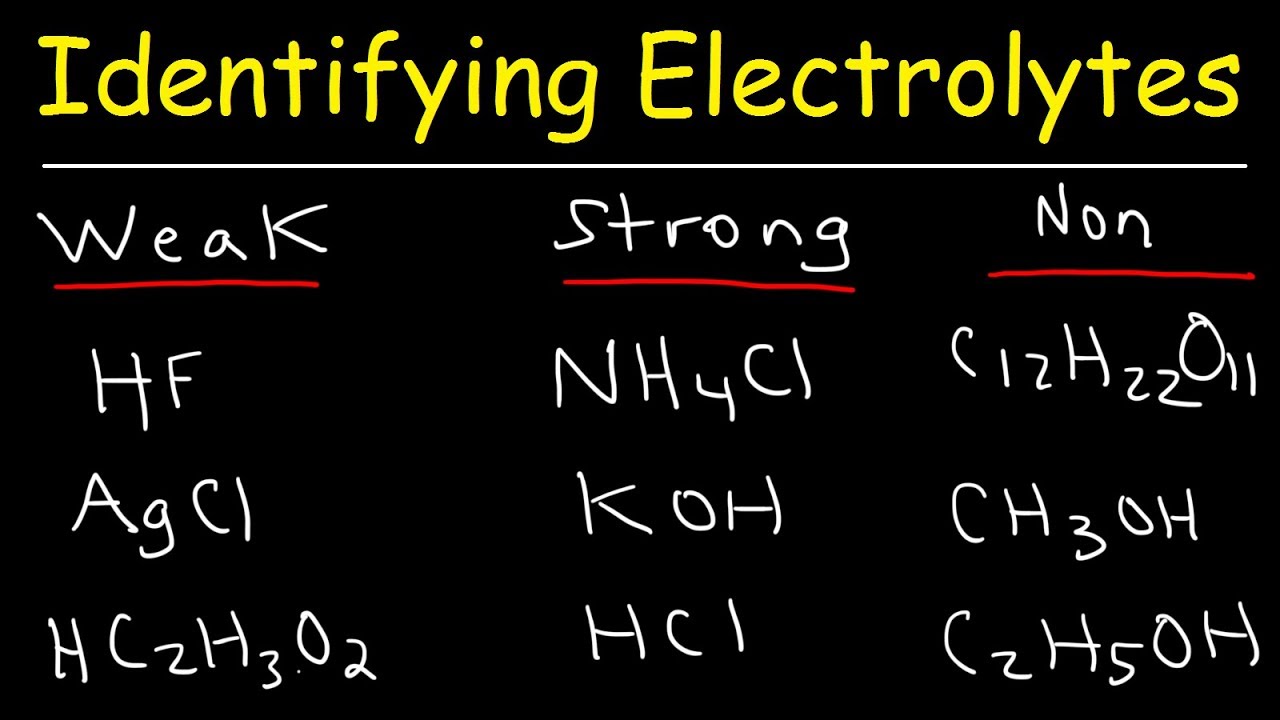
Identifying Strong Electrolytes, Weak Electrolytes, And Nonelectrolytes - Chemistry Examples - Youtube

Chapter 12 Solutions 12.2 Electrolytes And Nonelectrolytes - Ppt Download