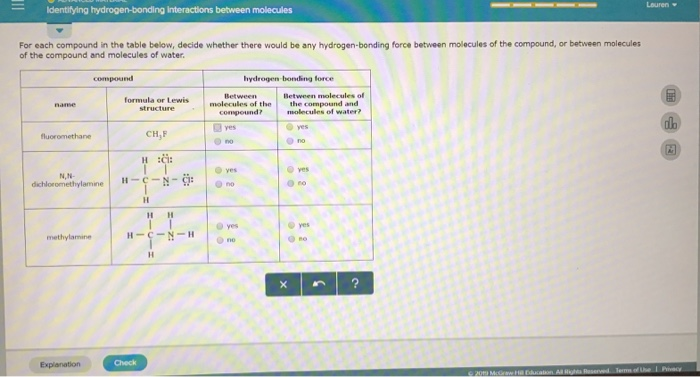
According to earlier definitions “hydrogen bonds is an interaction between the covalent pair a—h (donor) to a nearby electronegative atom b or x (acceptor). As we saw in the lewis structure of ch3f(fluoromethane), fluorine is bonded with carbon central atom, and hydrogen is attached only with carbon, not with fluorine.
Solved Louren Identifying Hydrogen-Bonding Interactions | Chegg.com
Basic structure of intermolecular hydrogen.

Is fluoromethane hydrogen bonding. And “a” is more electronegative [1] [2]. So, in order to the occurrence of hydrogen bond fluorine must be attached with hydrogen. However, ethane has a boiling point of 184.5c and fluoromethane has a boiling point of 194.7c.
You may find it useful to draw lewis structures for some of these molecules: Finally, there is a dipole formed by the difference in electronegativity between the carbon and fluorine atoms. When hydrogen is bonded to oxygen, like in h2o, the oxygen has a lot stronger pull on the electrons.
Furthermore, the molecule lacks hydrogen atoms bonded to nitrogen, oxygen, or fluorine; Effectively, in a covalent bond, the atoms share electrons. That is why ch3f does not have hydrogen bonding.
Which answer best explains this 10 degree difference in boiling point in terms of the van der waals forces present. Ethane (ch3ch3) and fluoromethane (ch3f) have the same number of electrons and are essentially the same size. Why does ch3f not have hydrogen bonding?
Hydrogen bonding is a weak (relatively) type of bond that occurs between two hydrogen atoms that are covalently bonded to a very electronegative atom such as flourine, oxygen, and nitrogen.

1.8: Intermolecular Forces - Chemistry Libretexts
Crystals | Free Full-Text | Rch3···O Interactions In Biological Systems: Are They Trifurcated H-Bonds Or Noncovalent Carbon Bonds? | Html