It facilitates reactions that follow polar mechanisms, such as sn2 reactions. A nash dmf sh b question :
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents
Our er nets the next sees polar protic for luck opera, teak and solve words.

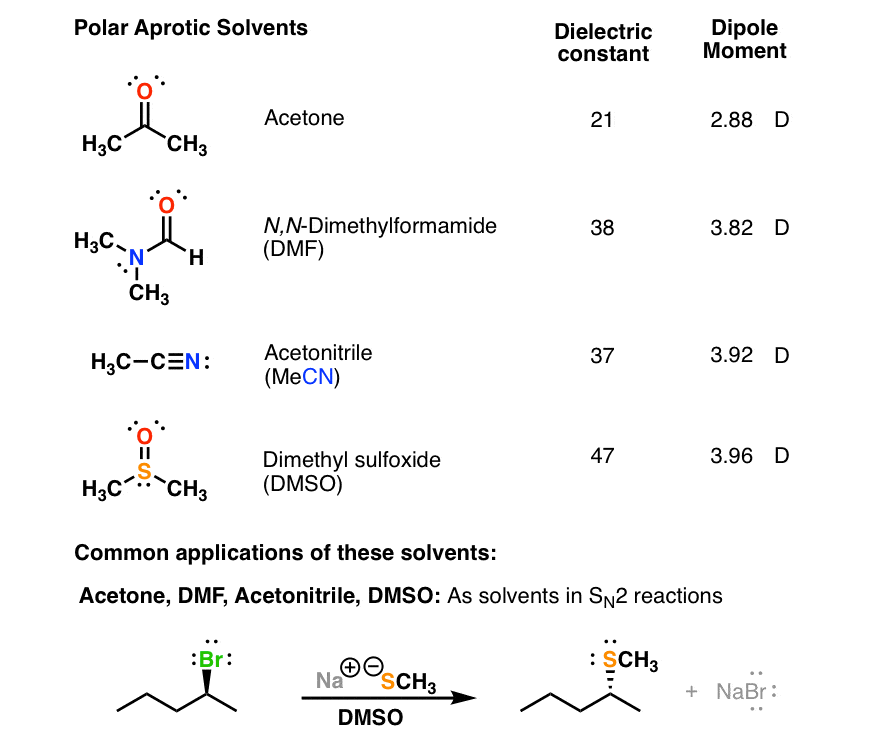
Is dmf polar protic. All three of them have bond dipoles but none of them have hydrogen attached to oxygen or nitrogen. Learn vocabulary, terms, and more with. For example, acetone ch3−co−ch3 ,.
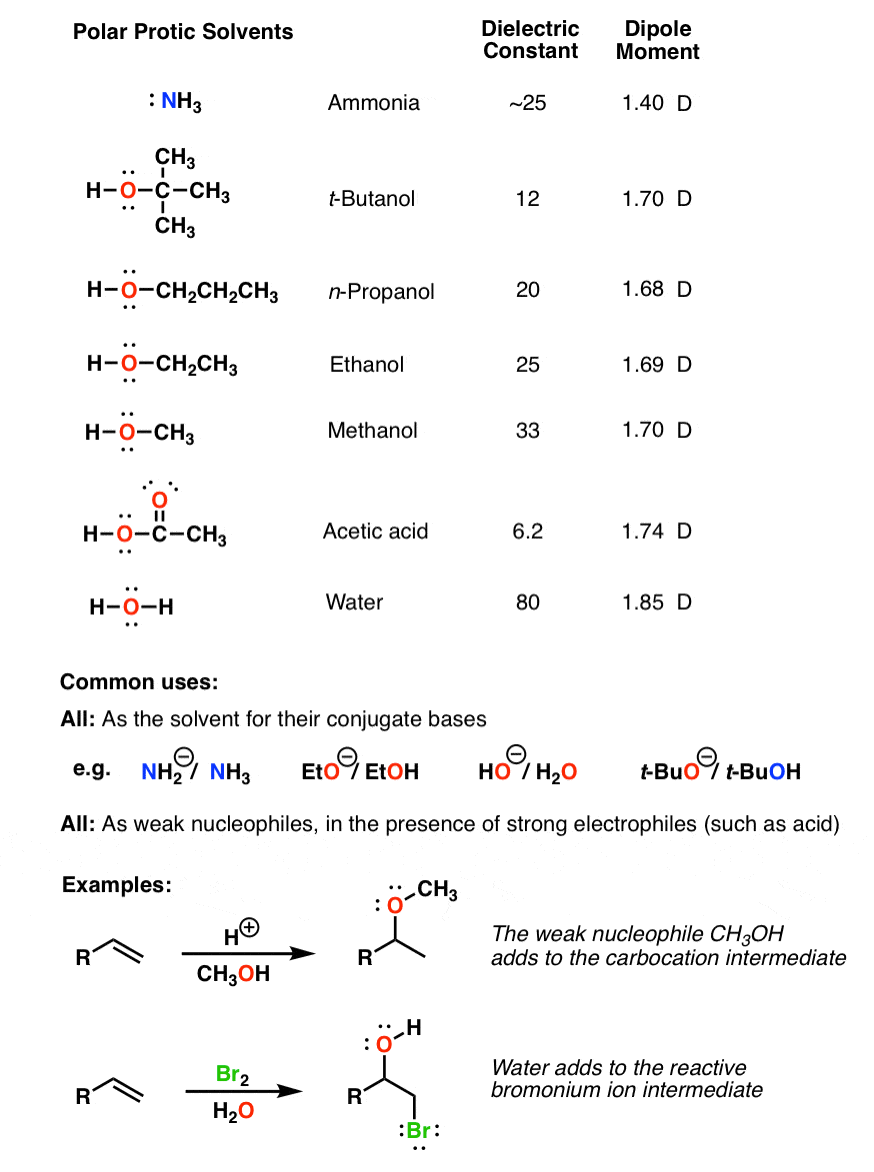
It is essentially odorless and has a low level of toxicity. Polar protic solvents are solvents that are capable of hydrogen bonding with the substrate in them. Polar protic solvents are capable of making hydrogen bonding i.e.
Start studying polar aprotic solvents. Polar aprotic solvents eg dmso acetone acetonitrile dmf are very effective in from organic ch 307 at rutgers university Uh, they farms hydrogen bonding, hydrogen bonding due to presence off hrm age, you need to do presents off or eight.
A) ether c) ethanol d) hexane b) dimethylformamide f) circle the correct name for the molecule shown below. They are highly polar on. Best example in this case is i sit on d m s o bcm.
Dimethyl sulfoxide (dmso) is a highly polar and water miscible organic liquid. So, we can say that all of them are polar aprotic solvents. A protic solvent is a solvent that has a hydrogen atom bound to an oxygen (as in a hydroxyl group), a nitrogen (as in an amine group), or fluoride (as in hydrogen fluoride).
I trialed eggs, edra, ex cetera. The molecules of such solvents readily donate protons (h ) to solutes, often via hydrogen bonding. Dimethylformamide is a polar aprotic solvent because it is a.
In general terms, any solvent that contains a labile h is called a protic solvent. Is dmf a polar protic solvent dmf is a polar (hydrophilic) aprotic solvent with a high boiling point. Hexamethylphosphoric triamide (hmpa) polar aprotic.
Polar aprotic solvents contain no hydrogen atoms connected directly to an electronegative atom and they are not capable of hydrogen bonding. You know they're highly polar. Conversely, polar aprotic solvents cannot donate protons but still have the ability to dissolve man…
However, polar aprotic solvents are those polar solvents which have no such acidic hydrogens. Is dmf polar protic or aprotic? So, dimethylformamide is a polar aprotic solvent.
Water is the most common protic solvent. 3 e) which solvent is a polar protic solvent? Start studying protic vs aprotic solvents.
An important property of protic solvents is that they give hydrogen gas on reduction which is highly useful in reductive chemistry. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Dimethyl sulfoxide (dmso) polar aprotic.
Jan 17, 2015 dimethylformamide is a polar aprotic solvent because it is a polar molecule and has no oh or nh groups.

The Role Of Solvent In Sn1, Sn2, E1 And E2 Reactions - Chemistry Steps