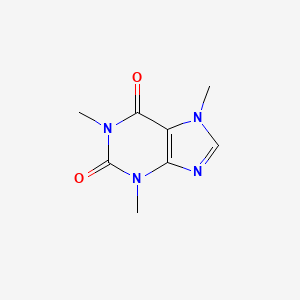
The atom with a higher electronegativity will have a slightly more negative charge due to the attraction of more electrons, causing an unequal sharing of electrons. Yes, caffeine is a polar molecule.
Is The Caffeine Molecule Polar Or Non Polar? | Socratic
Do you mean if caffeine, as a chemical molecule, is polar, and along which axis with which pole at what end?
Is caffeine polar or nonpolar. 3 more posts from the chemhelp community 45 posted by This is the exact definition of polarity itself. The oxygen and nitrogen molecules have a stronger polarity than carbon, allowing them to slightly pull the electrons towards them in their covalent bond.
If you’re looking for a more chemically accurate answer, the reason is because in the caffeine molecule, the nitrogen and oxygen are more strongly polar than the carbon, which allows them to more firmly pull the covalent electrons from the carbon counterparts. Allowing them to slightly pull the electrons towards them in their covalent bond. Caffeine (c8h10n4o2) is a polar molecule.
Polarity, like (almost, depends on definition as i recently discussed with someone on quora) every aspect of chemistry is related to. This will give those atoms a slightly negative charge while giving the carbon a positive charge. This will give those atoms a slightly negative charge while giving the carbon a positive charge.
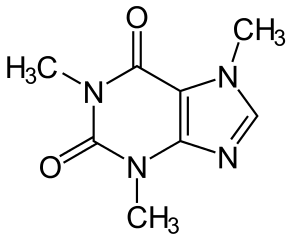
As you can see from its structure here: It's pretty clear that caffiene is more polar. However, caffeine has been found to be more polar than aspirin, according to an article published in the journal chemical science.
This will give those atoms a slightly negative charge while giving the carbon a positive charge. The oxygen and nitrogen molecules have a stronger polarity than carbon. Caffeine is indeed more polar, on top of your observations in hplc, you can also see this in how soluble each is in water.
Is caffeine polar nonpolar or ionic why do you conclude this way? No single molecule is entirely nonpolar or entirely polar. Allowing them to slightly pull the electrons towards them in their covalent bond.
The oxygen and nitrogen molecules have a stronger polarity than carbon. Answer #1 yes, caffeine is a polar molecule. Polar molecules typically have one or more highly electronegative atoms surrounded by.
In fact you can call caffeine both polar and nonpolar, as caffeine (or. Yes, caffeine is a polar molecule. Caffeine is water soluble, so it is a polar compound.