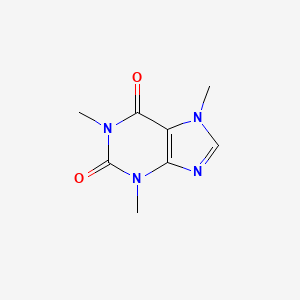
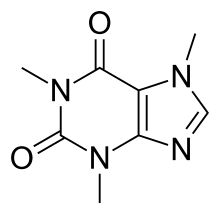
As you can see from its structure here: This will give those atoms a slightly negative charge while giving the carbon a positive charge.
Caffeine | C8H10N4O2 - Pubchem
Caffeine is water soluble, so it is a polar compound.
Is caffeine non polar. 3 more posts from the chemhelp community 45 posted by Yes, caffeine is a polar molecule. However, caffeine has been found to be more polar than aspirin, according to an article published in the journal chemical science.
The atom with a higher electronegativity will have a slightly more negative charge due to the attraction of more electrons, causing an unequal sharing of electrons. This is the exact definition of polarity itself. Caffeine is a polar molecule.
Yes, caffeine is a polar molecule. The oxygen and nitrogen molecules have a stronger polarity than carbon, allowing them to slightly pull the electrons towards them in their covalent bond. Paracetamol may interact with other apis or excipients when used together, e.g., many drug formulations including paracetamol and caffeine irrespective of the fact that both.
This is a simplification, since caffeine is large enough that the polarity of individual (or a few) bonds matters as well. Aspirin is less polar than caffeine and acetaminophen because of the ether, but it is more polar than ibuprofen. But let’s leave that for now.
Caffeine is indeed more polar, on top of your observations in hplc, you can also see this in how soluble each is in water. No single molecule is entirely nonpolar or entirely polar. It is a stimulant and the form i of caffeine is metastable.
In tlc, caffeine has the lowest rf value when the stationary phase is silica and caso4, and the mobile phase is butyl ethanoate and trichloroethane. Peter fink’s links seem to confirm this. As you can see from its structure here:
This will give those atoms a slightly negative charge while giving the carbon a positive charge. This is the exact definition of polarity itself. That, or sublimation, but i've never tried that.
It's pretty clear that caffiene is more polar. The oxygen and nitrogen molecules have a stronger polarity than carbon, allowing them to slightly pull the electrons towards them in their covalent bond. And the polarity runs (in the image above) approximately from the right/lower right (being the more positive) to left/upper left (being more negative).
As you can see from its structure here: Yes, caffeine is a polar molecule. National center for biotechnology information.
The oxygen and nitrogen molecules have a stronger polarity than carbon, allowing them to slightly pull the electrons towards them in their covalent bond.this will give those atoms a slightly negative charge while giving the carbon a positive charge. Allowing them to slightly pull the electrons towards them in their covalent bond. But in any case, caffeine is not a very polar molecule (if it were it would not partition favorably from water into chcl3), and i suspect it is much more soluble in chcl3 than in etoh.
Yes, caffeine is a polar molecule. Acetaminophen will have rf value of about 0.4 and ibuprofen will have rf value of about 0.6 if the non polar solvent used is ethanol. One important application of this.
The oxygen and nitrogen molecules have a stronger polarity than carbon.