The hydrogens bond with the two carbons to produce molecular orbitals just as they did with methane. Whereas methanol is infinitely miscible with water, which of course is strongly hydrogen bonding.

Hydrogen Bonding With A Hydrogen Bond: The Methane–Water Complex And The Penta-Coordinate Carbon - Sciencedirect
Sulfur is heavier than oxygen,.
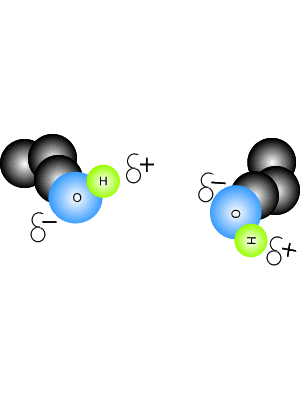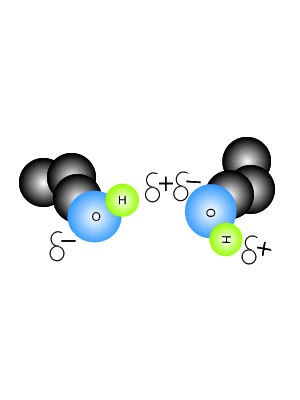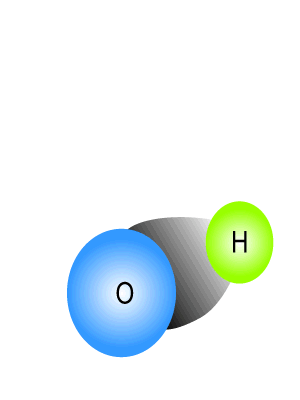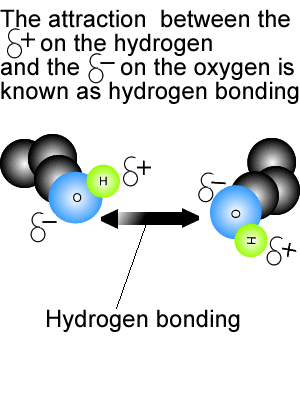
Hydrogen bonding force of methane. Video answer:we have been given the methane and we have been asked that there is hydrogen bonding or not between the molecules. Video answer:we have been given the methane and we have been asked that there is hydrogen bonding or not between the molecules. Such a bond is weaker than an ionic bond or covalent bond but stronger than van der waals forces.
The best known example of hydrogen bonding is water: Hydrogen bonds can exist between atoms in different molecules or in parts of the same molecule. On the other, other hand, phenol has limited water solubility;
Hydrogen bonding is observed when h is present in a compound with a highly electronegative element like f, o, and n. For example, water melts at 0.00 °c and boils at 99.98 °c; Hydrogen bonding in water vs hydrogen sulfide.
Kolade emmanuel omolagba studied sss 3 (graduated 2014) 1 y related There is no hydrogen bonding between meeting molecules and there is no hydrogen bonding of methane with water. 100% (12 ratings) transcribed image text:
Experts are tested by chegg as specialists in their subject area. The basic condition to form hydrogen bonds is that hydrogen should be attached to a highly electronegative element like nitrogen, oxygen or fluorine or any other highly electronegative element. No hydrogen bonding with water because it is a non polar molecule while in enclosed metal.
Since in chcl3 the hydrogen is not attached to fluorine, oxygen or nitrogen so people usually think that chloroform does not form hydrogen bonds. In this case, h is present along with c. Sulfur is in group 16 of the periodic table, the same as oxygen.
Hydrogen bonding is abscent in methane.hydrogen bonding is present between highly electronegative elements like fluorine,oxygen,nitrogen and hydrogen.examples for hydrogen bonding are water [between hydrogen and oxygen],hydrogen fluoride [between hydrogen and fluorine] etc. Hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; It is alsovital that you refer to the hydrogen bonding as being between molecules and not within them.
No hydrogen bonding with water because it is a non polar molecule while in enclosed metal. What is the intermolecular force of ch3cooh? We review their content and use your feedback to keep the quality high.
National center for biotechnology information. On the other hand, the boiling point of methanol has increased over 200 ∘c with respect to its parent hydrocarbon, whereas for phenol, the equivalent increase is 100 ∘c. There is no hydrogen bonding between meeting molecules and there is no hydrogen bonding of methane with water.

What Type Of Intermolecular Forces Are Present In Ch4? | Homework.study.com

Aleks - Identifying Hydrogen Bonding Interactions Between Molecules - Youtube