Bromine compound is a molecule formed when two bromine atoms combine together. How many valence electrons does br have and how many more does it need to fill a from science 1516 at university of canberra

How Many Electrons Are In The P Sublevel In The Br Atom
Valence describes how easily an atom or a free radical can combine with other chemical species.
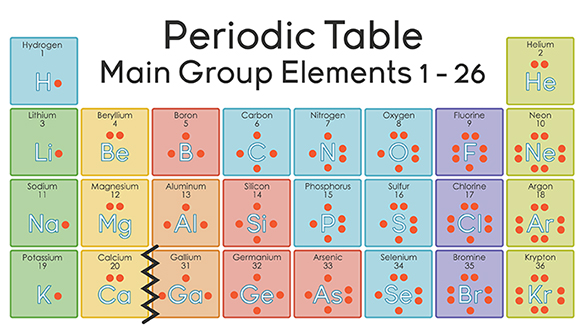
How many valence does br have. Theblogy.com » br has how many valence electrons. 1 what does br stand for? Thus, bromine has seven valence electrons.
Valence electrons in strontium (sr) 2: How many valence electrons does brf2 have? Therefore, the valence electrons of bromine are seven.
Valence electrons are the outermost electrons in an atom, which affect how atoms might react with one another. Valency of bromine (br) there are many different ways to find out the valency of an atom which reflects the ability of an atom to bond with other atoms. Valence electrons in yttrium (y) 3:
They are the electrons involved in the bond formation. To know these properties of bromine one must know the number of electrons and protons of bromine. Valence electrons in krypton (kr) 8:
How many valence electrons does ar have, and what are the specific valence electrons for ar? 18 how many protons does br have? All noble gases have eight valence electrons.
How many valence electrons does the element br have? Valence electrons in rubidium (rb) 1: A halogen gas used for bleaching textiles and.
A bromine (br) atom has 7 valence electrons. Step 1 1 of 8. Reference wikipedia farhan sadik quality education can build a beautiful society.
Oxygen has six electrons so it cannot bond with any atoms that have less than six electrons including hydrogen because they don’t share enough in common to form a bond. Last updated on july 22, 2022 by amin. Valence electrons are the electrons found in the outer most shell of an atom.
Since bromine has 7 valence electrons the 4s orbital will be completely filled with 2 electrons and the remaining five electrons will occupy the 4p orbital. Bromine is a chemical element with the symbol br and atomic number 35. 19 how to find the valence electrons for bromine (br) 20 is.
How many valence electrons does br have, and what are the specific valence electrons for br? Br has how many valence electrons. 119 rows valence electrons in bromine (br) 7:
What is the valency of br2? The valence electrons of bromine are 2, 8, 18, 36, 54. Step 2 2 of 8.
Number of valence electrons = (express your answer as a series of orbitals in order of increasing orbital energy.) specific valence electrons: How many valence electrons does bromine (br, atomic no. Valence electrons in niobium (nb) 5:
To quickly determine the number of. Valence electrons in zirconium (zr) 4:

Br Bromine Element Information: Facts, Properties, Trends, Uses And Comparison - Periodic Table Of The Elements | Schoolmykids
What Is The Number Of Valence Electrons In Nitrogen? | Socratic