School university of north florida; That would mean that it has gone through 10,000 half lives.

6. How Long Is Material Radioactive? Carbon-14 Archaeological Dating, Half- Life Uses, Decay Curve, Geological Dating Of Igneous Rocks, Radiocarbon-14 Dating, Long Term Storage Problems Of High Level Nuclear Waste Ks4 Science Igcse/Gcse
This equation is used in the calculator when solving for.
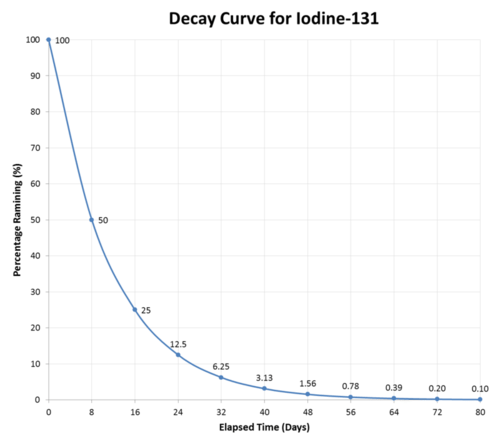
How many half lives to completely decay a mole. Some chemical isotopes contain unstable nuclei that will spontaneously decay over time and emit radiation. The number of half lives needed for a radioactive. This is an exponential decay, as seen in the graph of the number of nuclei present as a function of time.
That continues leaving less and less molecules of the original one mole. Mahesh prakash author has 4.6k answers and 1.3m answer views jul 3 there is nothing special about 14c I.e., less than a 50% chance of even one atom remaining.
Ln (nt / no) = kt. The simple reason why the number of decays (strictly, the number of decays per unit time) decreases in simple radioactive decay is because there are fewer atoms left to decay. From the start of timing it takes two days for the count to halve from 80 down to 40.
So, in terms of moles, starting with one mole, after one half life, you have 0.50 moles. Suppose you get down to one molecule remaining. The probability of any given unstable atom decaying is constant (independent of time or the environment).
Note that this second two. 38 how many half lives are required for the concentration of reactant to. 38 how many half lives are required for the.
It takes another two days for the count rate to halve again, this time from 40 to 20. There is a direct relationship between radioactive decay and half life of a radioactive substance. Some isotopes of elements found in nature have unstable nuclei, meaning they naturally decay into more stable nuclei through various processes.
It has a halflife of 5700 years. The rate of radioactive decay is measured in half life equivalents. Round your answer to the nearest whole number.
The number of half lives needed for a radioactive element to decay to about 6 of. It seems like a long time. Calculate the number of nuclei.
From the above equation, we can derive another important equation for the calculation of the rate of radioactive decay. A sample containing this isotope has an initial activity (t = 0) of 60.0 muci. But, say we have a coal deposit that is 570 million years old.
One more half life and you have 0.25 moles. What is its decay constant? First of all, who cares?
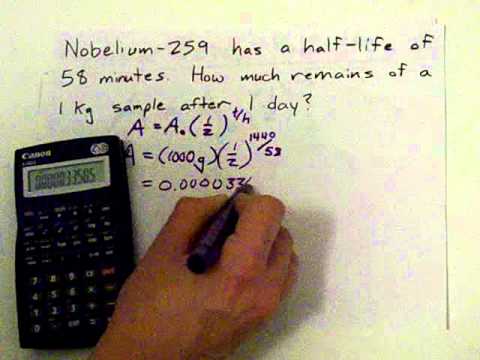
Half-Life Calculations: Radioactive Decay - Youtube
How Can We Explain The Half Life Of A Radioactive Element? Why Can't We Explain Radioactive Decay By Full Life? - Quora