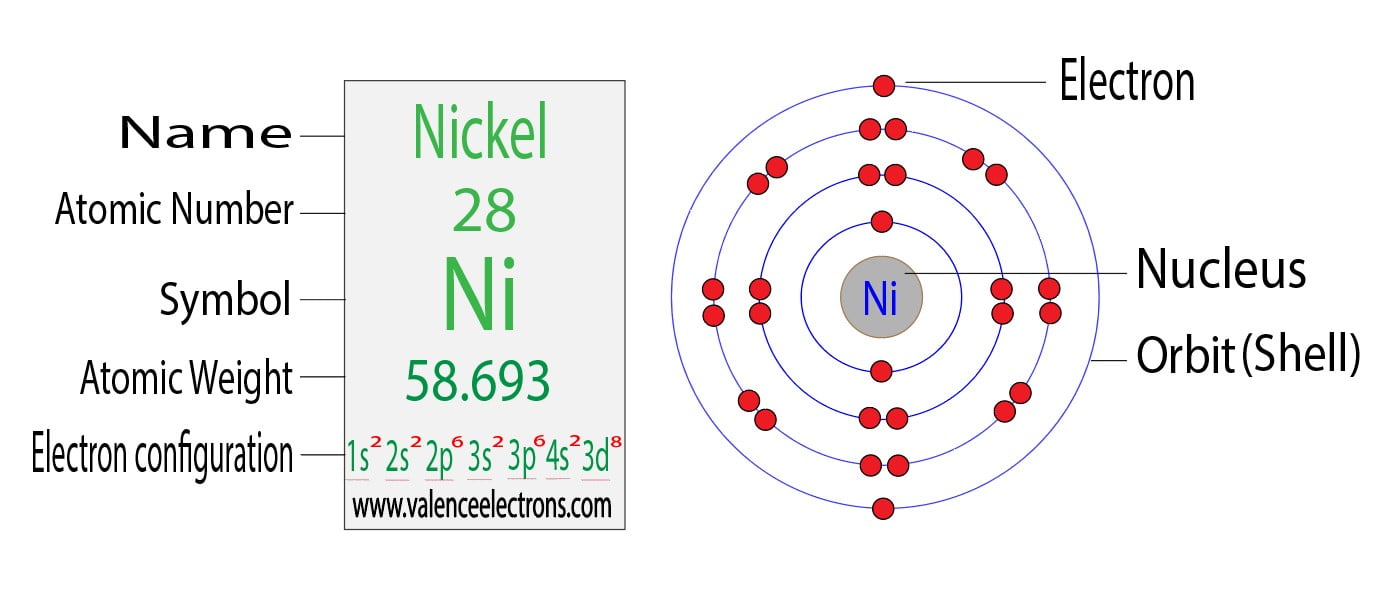
[ar], 3d8, 4s2 atomic number of nickel (ni) = 28 electronic configuration is 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d8 or [ar], 3d8, 4s2 where [ar] is argon with atomic number 18. Interactive periodic table let me tell you how this interactive periodic table will help you in your studies.
Nickel(Ni) Electron Configuration And Orbital Diagram
The first two electrons enter the 1s orbital and the next two electrons enter the 2s orbital.

Full electron configuration of nickel. Spectrum noir duo color markers spectrum noir duo color markers Either way, the nickel electron configuration will be 1s2 2s2 2p6 3s2 3p6 3d8 4s2 note that when writing the electron configuration for an atom like ni, the 3d is usually written before the 4s. 119 rows electron configuration chart of all elements is mentioned in the table below.
So, six electrons enter the 2p orbital. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the table. The editors of encyclopaedia britannica
July 7, 2022 post category: Chemistry electron configuration electron configuration. Full electron configuration of nickel:
1 answer sarita rana feb 9, 2017 Cobalt ← nickel → copper. Custom right text here fossil fuel consumption per capita by country 2021.
1s 2 2s 2 2p 6 3s 2 3p 6 3d 8 4s 2. 1s2 2s2 2p6 3s2 3p6 3d8 4s2. Such an electron configuration results in elevated light response, conductivity, charge separation, and mobility of the photocatalyst concurrently, thus largely augmenting the photocatalytic.
76ers vs thunder last game post comments: What is nickel's electron configuration? [ar]4s13d9 (four nickel is a transition metal, with an atomic weight of 28, located in the fourth period/row on the periodic table of elements the 3d orbital of the partially oxidized ni single‐atom sites is filled with unpaired d‐electrons, which are ready to be excited under irradiation.
Full electron configuration of nickel. >> back to key information about the element. The next six electrons enter the 2p orbital.
The colourless, volatile liquid is formed by the action of carbon monoxide on finely divided nickel and is characterized by an electronic configuration in which the nickel atom is surrounded by 36 electrons. The electron configuration of nickel is:
How To Know That [Nicl4] 2- Has Tetrahedral Geometry Whereas [Ni(Cn) 4] 2- Has Square Planar Geometry - Quora

Electron Configuration For Ni, Ni2+, And Ni3+ (Nickel And Nickel Ions) - Youtube