Beco 3 → beo + co 2 be (oh) 2 → beo + h 2 o 2 be + o 2 → 2 beo igniting beryllium in air gives a mixture of beo and the nitride be 3 n 2. (11) m ( s) + o 2 ( g) → m o 2 ( s) where m represents sr or ba.

How To Balance Be + O2 = Beo (Beryllium + Oxygen Gas) - Youtube
From these sources, beryllium is emitted into the air and water by natural processes like erosion and by the burning of coal and oil.

Formation of beryllium from oxygen. Valle published 19 may 2005 physics astronomy and astrophysics we investigate the evolution of the star formation rate in the early galaxy using beryllium and oxygen abundances in metal poor stars. Abstract previous workers 1 have concluded from a comparison of the reaction between beryllium in dry carbon dioxide at 650° c. Beryllium is a hard, grayish metal naturally found in mineral rocks, coal, soil, and volcanic dust.
Beryllium oxide can be prepared by calcining (roasting) beryllium carbonate, dehydrating beryllium hydroxide, or igniting metallic beryllium : Beryllium is reluctant to burn unless it is in the form of dust or powder. Therefore, beryllium is a cation element.
And in dry oxygen at 650° c. Specifically, we show that stars belonging to two previously. Formation of simple oxides on the whole, the metals burn in oxygen to form a simple metal oxide.
Department of energy's office of scientific and technical information Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.) granted application number gb8024304a other versions gb2054542b (en We investigate the evolution of the star formation rate in the early galaxy using beryllium and oxygen abundances in metal poor stars.
Both reactions require heat and excess oxygen. First, its low mass makes it a convenient substrate for the detection of oxygen and nitrogen by means of. Beryllium beryllium is unreactive with air and water.
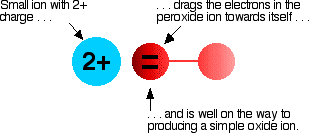
Early star formation in the galaxy from beryllium and oxygen abundances l. Early star formation in the galaxy from beryllium and oxygen abundances @article{pasquini2005earlysf, title=early star formation in the galaxy from beryllium and oxygen abundances, author={luca pasquini and daniele galli and raffaele gratton and piercarlo bonifacio and sofia randich and g. Beryllium leaves two electrons and turns into a positive ion.
Beryllium has a very strong (but very thin) layer of beryllium oxide on its surface, and this prevents any new oxygen getting at the underlying beryllium to react with it. The general reaction is given below: These two components di er not only in their kinematical properties, but also in their chemical composition(see g03).
According to data collected by the environmental protection agency (epa), the average concentration of airborne beryllium in the u.s. Bonding of beryllium to four oxygen atoms with the formation of beo4 tetrahedra is also exclusive for its natural occurrence. Be 2c is present only at low fluences and before the equilibrium is established.
Cases with be in coordination higher than four have not been observed. This metal has been chosen as a model system for the following reasons. Of a detailed study of the formation of nitrides and oxides on beryllium.
Under kinetic conditions that the higher. Beryllium and oxygen in the early galaxy the accretedpopulation rst proposedby searle & zinn (1978) to explain the formation of the halo. Roth et al [ 12 ] studied the oxidation kinetics of vacuum hot pressed beryllium samples heated in oxygen at various temperatures by measuring the oxide thickness with rutherford backscattering (rbs).
The first is the chemisorption of oxygen on a clean beryllium surface, the second the logarithmic growth of very thin oxide films, limited by electron tunnelling. The elements that form bonds by donating electrons are called cation.