Nitrogen, which makes up about 78% of our atmosphere, is a colorless, odorless,. 1s 2 2s 2 2p 3:

Electron Configuration For Nitrogen Ion - Periodic Table
Many industrially important compounds, such as ammonia, nitric acid, organic nitrates.
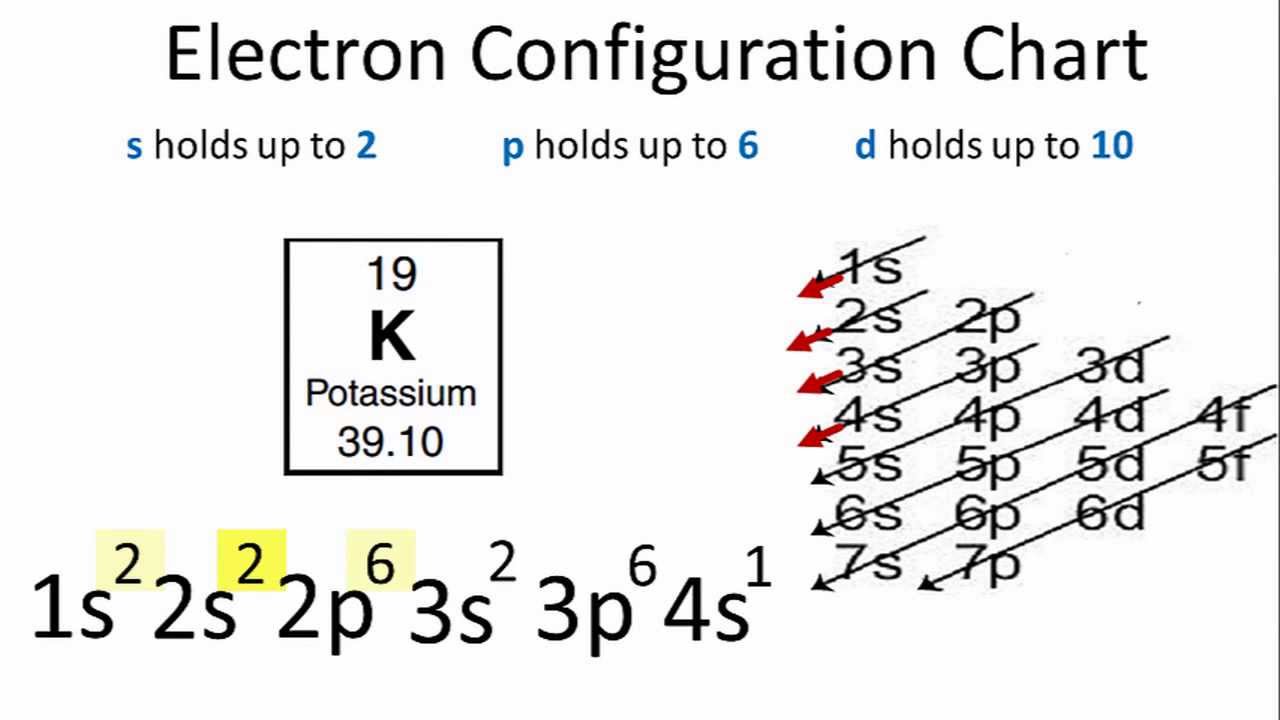
Electron configuration of nitrogen ion. The first three ionisation energies of nitrogen are 1.402, 2.856, and 4.577 mj·mol −1, and the sum of the fourth and fifth is 16.920 mj·mol−1. Electron configuration chart of all elements is mentioned in the table below. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital.
As sulfur has an atomic number of 16 a neutral atom… skip to content The electronic configuration of each element is decided by the aufbau principle which states that the electrons fill orbitals in order of increasing energy levels. When we talk about school subjects, then one of the major subjects which are very important for knowledge perspective is science.
The period or row numbers 1 through 7 are the energy levels of the elements. Atomic number is 29 expected atomic number is 1s2 2s2 2p6 3s2 3p6 4s2 3d9 or [ar] 4s2 3d9 here, 3d orbital has 9. Due to these very high figures, nitrogen has no.
If you are still not getting the nitrogen electron configuration of the element nitrogen then, the full electronic configuration of nitrogen is written as the following; The shorthand electron configuration (or noble gas configuration) as well as full electron. This electron configuration of nitrogen.
Medium solution verified by toppr this is the electron configuration of nitrogen ion (n −3). The electronic configuration of nitrogen is 1s 2 2s 2 2p 3. Hence, the electron configuration for ca 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6.
· priya mar 10, 2014 the nitride ion is n −3 the original electron configuration for nitrogen is 1s22s22p3 in order to fulfill the octet rule, the nitrogen atom would. Since we need to take away two electrons, we first remove electrons from the outermost shell (n=4). We’ll also look at why nitrite forms.
Electron configuration of nitrogen is [he] 2s2 2p3. The p orbital can hold 6. Electronic configuration of nitrogen is 1s2 2s2 2px1 2py1 2pz1 copper:
The s orbital holds a maximum of 2 electrons. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. We know, the electron configuration of the nitrogen atom is 1s 2 2s 2 2p 3, and valence electrons are those electrons found in the outer shell of an atom.
This element has a great capacity to create triple bonds when combined with another nitrogen atom and with other types of. The d orbital can hold 10. What is the electron configuration of a nitrogen ion?
In order to write the n electron configuration we first need to know. The correct electron configuration of the ion formed by sulfur will be 1s2 2s2 2p6 3s2 3p6. And so the electron configuration here for calcium with a positive two charge, this calcium cation, is.

Webelements Periodic Table » Nitrogen » Properties Of Free Atoms
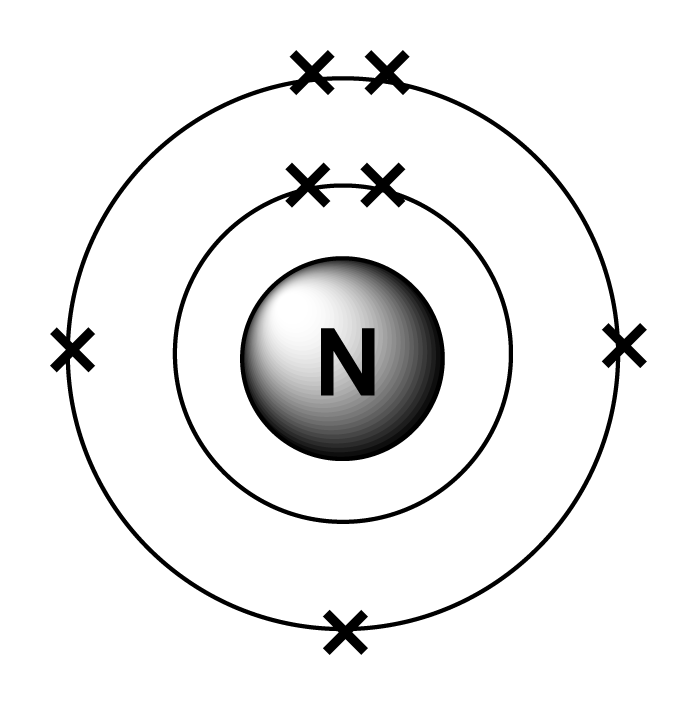
Explanation Of Nitrogen Ionizations | Lefteris Kaliambos Wiki | Fandom