What is the electron configuration of au+ ? If you want to be 100% sure search for a graph that shows orbitals and the energy associated with them.
Solved Write The Ground-State Electron Configurations For | Chegg.com
In order to write the au electron configuration we first need to know the number of electrons for the i atom (there are 79lectrons).

Electron configuration for au+. For example, calcium is element 20. For example, [he]2s22p2 would be entered as [he]2s^22p^2 expert. So the number of electron must be 15 , right ?
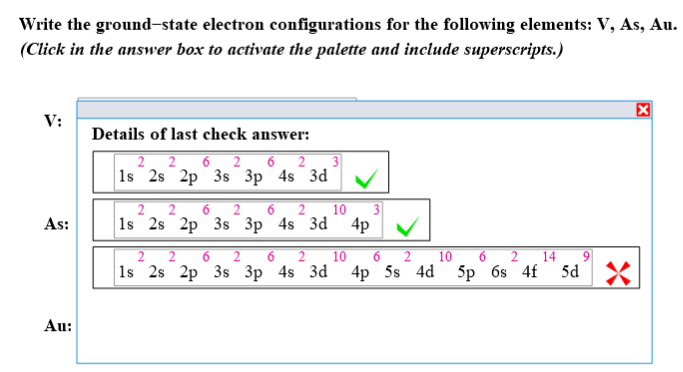
Electron configuration for phosphorus is 1s2 2s2 2p6 3s2 3p3. This only applies to the first row transition. Vanadium (v) #v# is atomic number #23#, so its configuration is #1s^2 2s^2 2p^6 3s^2 3p^6 3d^3 4s^2#.in shorthand it is #[ar] 3d^3 4s^2#.this is an expected.
Electron configuration of hydrogen (h) 1s 1: The ion au+ electronic configuration is : Does it effect the number of electron ?
1 level 1 · 7 yr. [(xe)], 4f14 , 5d10, 6s0 Get 20% off grade+ yearly subscription →
Shorthand electron configuration full electron configuration electron shell arrangement; But , what if m = 1 ? Medium solution verified by toppr the atomic number of au is 79.
Gold is a classified transition metal element. Electrons enter in that subshell first which has the least value of. When we write the configuration, we'll put all.
This decides the electron capacity of the shells. The atomic number of au is 79. 1s 22s 22p 63s 23p 63d 104s 24p 64d.
Nevertheless, check the complete configuration and other interesting facts about gold that. [xe] 4f14 5d10 6s1 the electron. Predicted data is generated using the us environmental protection agency s episuite™.
There are different electron configurations for gold because gold is a transition metal. A)write electron configurations for au+ express your answer in condensed form, in order of increasing orbital energy. First, find the required element on the periodic table.
In the case of gold the abbreviated electron configuration is [xe] 4f14 5d10 6s1. The standard (noble gas) electron configuration is : Gold (au) electron configuration and orbital diagram gold is the 79th element in the periodic table and its symbol is ‘au’.
If you want to do manually then follow the steps below to write shorthand electron configurations: Log kow (kowwin v1.67 estimate) = 0.73 boiling pt,. Remember the + means it has lost 1 electron, your configuration looks like au has gained an electron instead reply more posts you may like r/chemhelp• electron configuration of.
There were three rules for electronic configuration. The electron configuration for the first row transition metals consists of 4s and 3d subshells with an argon (noble gas) core. Ago calc or chem but really anything the d block is in a higher.
If the configuration is a noble gas, enter the noble gas in brackets, for example [ne] chemistry.

Solved Which Is The Correct Electron Configuration For Au+? | Chegg.com

Chemistry Chapter 15 Ionic Bonding And Ionic Compounds - Ppt Download