The lewis structure for c o 2 has two double bonds between the carbon and the oxygen. Each of the oxygen atoms has two lone pairs around it.
Solved Several Resonance Structures For The Molecule Co2 Are | Chegg.com
Does co2 have resonance structures?
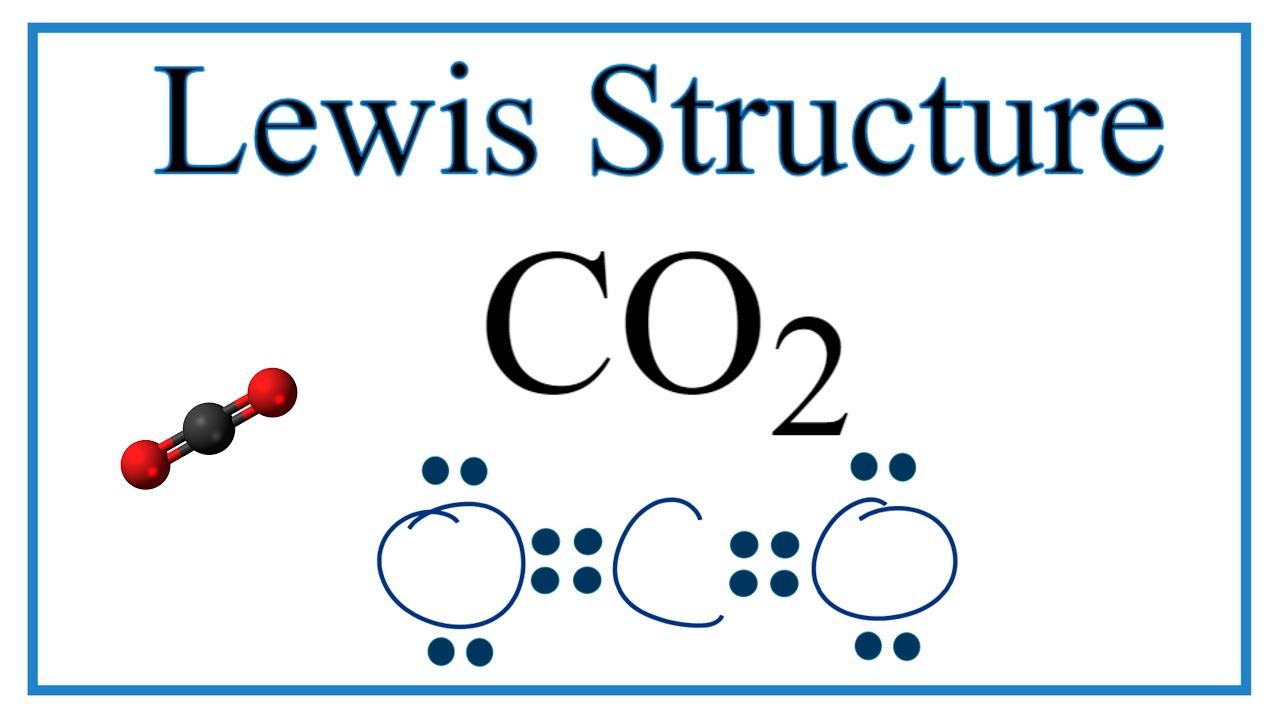
Does co2 have resonance. The actual structure is an average of these three resonance structures. The best and most stable configuration for co2 has two double bonds on each oxygen without any formal charge on carbon. We start with a valid lewis structure and then follow these general rules.
Resonance structure of butadiene explains h2c. The reason for resonance is to move the formal charge onto other atoms. For the co2 resonance structure there is only one major.
Does co 3 have resonance? The actual structure is an average of these three resonance structures. The carbonate (co2−3) ion unlike o 3, though, the actual structure of co 3 2 − is an average of three resonance structures.
In case of c o x 2 you could imagine a resonance structure in which the carbon doesn't have an electron octet and a positive charge and one of the oxygen atoms carrying a negative charge. The carbonate (co2−3) ion unlike o 3, though, the actual structure of co 32−is an average of three resonance structures. Feb 7, 2015 carbon dioxide, or co2, has three resonance structures, out of which one is a major contributor.
This is a question our experts keep getting from time to time. Does carbon monoxide have resonance lewis structures? Does co2 have resonance structures?
This completes the valence of 4 for the carbon. 18) the triple bond present in diatomic nitrogen, n2, is what makes this molecule so reactive. Here are the three resonance structures for co2, all accounting for the 16 valence electrons
If so, why and how? Co2 resonance structure of butadiene? Since carbon has no formal charge, the bonds do not resonate.
You could also draw one in which the carbon is only connected via a single bound to each oxygen. Carbon has 4 valence electrons, each oxygen has 6 valence electrons, and there are 2 more for the −2 charge. Is co2 3 a resonance structure?
What do resonance structure represent? It is almost always possible to draw resonance structures. This is known as carbon and hydrogen.
There are three resonance structures co2 (carbon dioxide). Now, we have got the complete detailed explanation and answer for everyone, who is interested! Carbon dioxide, or co2 , has three resonance structures, out of which one is a major contributor.
Carbon has 4 valence electrons, each oxygen has 6 valence electrons, and there are 2 more for the −2 charge.

Resonance Structures For Co2 (Carbon Dioxide) - Youtube
Chemistry Net: Simple Procedure For Writing Lewis Structures- Co2, Nco- – Examples #2

