Due to this unsymmetrical structure and electronegativity difference between nitrogen and hydrogen nh 3 possesses a strong permanent dipole moment of 1.4d. Which of the following molecules does not have a net dipole moment?
What Is The Reason H2O Has More Dipole Moments Compared To Nh3? - Quora
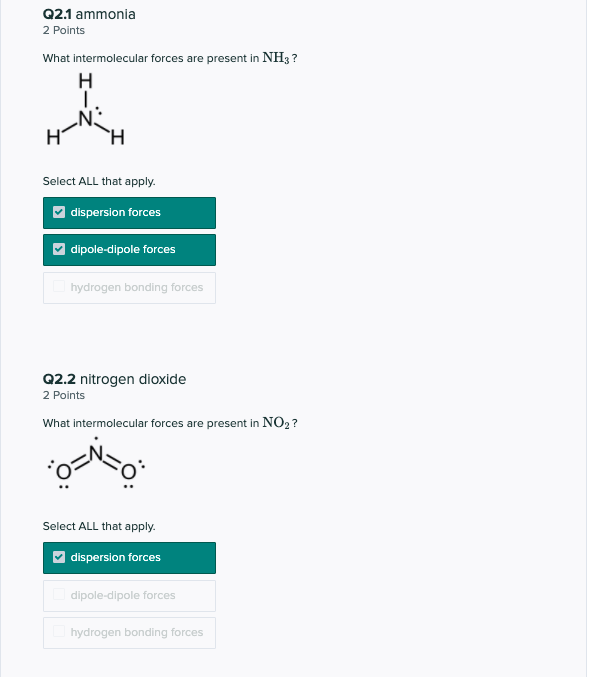
In case of nh3, the main type of intermolecular forces is;

Does ammonia have dipole dipole forces. Likewise know about, types, polarity and faq. Does ccl4 have a higher boiling point than ch2cl2? Which of the following molecules does not have a net dipole moment?
Does h2s have a dipole moment? Ammonia (nh3) in ammonia, the central atom nitrogen is sp 3 hybridizes and attain a trigonal pyramidal geometry with lone pair on nitrogen. 2 reply start new discussion page 1.
Exposure can cause dizziness, poor sleep, headache, anxiety, anorexia, weight loss, and vision changes. Hello, reders welcome to an additional fresh article on “hunterriverpei.com“. Which of the following molecules does not have a net dipole moment?
Permanent dipole moment of ammonia. Does ccl4 have a higher boiling point than ch2cl2? Does nh3 have dipole dipole forces.
It can harm the eyes, kidneys, blood, heart, liver, nerves, and skin. Exactly the same with water. Which of the following molecules does not have a net dipole moment?
Ammonia (nh3) is make hydrogen bonding and it effect extensive hydrogen bonding between molecules. Below you understand around the nh3 intermolecular forces, what is the in reality intermolecular forces in between nh3 molecules. Workers may be harmed by carbon disulfide.
Solved Nh3 Dispersion Forces O Dipole-Dipole Forces • | Chegg.com
Solved Q2.1 Ammonia 2 Points What Intermolecular Forces Are | Chegg.com