Oxidation number rule 1 the sum of oxidation numbers of all the atoms in a molecule is equal to zero. The oxidation number of cu in cu (o2cco2) is +2.

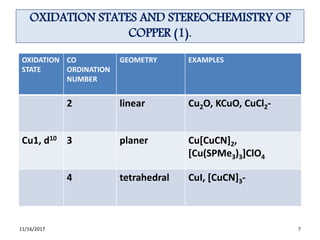
Combinations Of The Oxidation States For The Copper Complexes Observed [15] | Download Scientific Diagram
Therefore we know that the sum of all the oxidation numbers of cu, n, and o is equal to 0.
Cu oxidation number. In cuh the hydrogen has o.n. +1 and the copper has o.n. But on the internet it is ubiquitously told that the o.n.
How to find the oxidation number for cu in the cus ion. Simply add the numbers based on the formula: Thus, we can conclude that the oxidation state of copper in is +1.
Since two hydroxide (oh − −) ions are required to cancel the. Therefore, calculate the oxidation state of copper as follows. Rules for assigning oxidation numbers).
5,168 views oct 23, 2020 to find the correct oxidation state of cu in cus (the copper (ii) sulfide ion), and each element in the ion, we use. There are several ways to work this out. Also, in the case of a neutral compound, the summation of the oxidation numbers of all the atoms or ions is zero.
Except in metal hydrides, which this is not, hydrogen always has an oxidation state of +1. To find the correct oxidation state of cu in cu (oh)2, copper (ii) hydroxide, and each element in the compound, we use a few rules and some simple math. The first step is to jot down the charges of the elements since it is an ionic bond.
🛠️ calculate oxidation numbers instruções use letras maiúsculas para o primeiro carácter no elemento e minúsculas para o segundo carácter. Thallium (i) tl +1 +1: I am confused which one is correct.
Mercury (i) hg 2 +2. The oxidation number of c in cu (o2cco2) is +3. First, since the cu (oh)2 doesn’t have an.
The oxidation number is synonymous with the oxidation state. Oxidation number method in basic medium part 2. The cu (no3)2 is an ionic compound with overall oxidation number “0”.
The first step is to jot down the charges of the elements since it is an ionic bond. Gold (i) au +1 +1: Of hydrogen is −1 in cuh too, and the compound's name is copper (i) hydride.
Fe, au, co, br, c, o, n, f. Ammonia in this complex is not an ion, it is a neutral structure covalently bound to the copper atom; 60 rows information of oxidation numbers of monoatomic ions in periodic table.
Copper (i) cu +1 +1: My chemistry teacher said hydrogen when bonded with metals has oxidation number (o.n.) of −1 except cuh: Therefore we know that the sum of all the oxidation numbers of cu, n, and o is equal to 0.
Thus having a net oxidation number of 0. When charge is negative then it means the element or atom has gained electrons. Oxidation number (also called oxidation state) is a measure of the degree of oxidation of an atom in a substance (see:
Therefore, the oxidation numbers of zn and cu are zero. Simply add the numbers based on the formula: It is known that the charge.
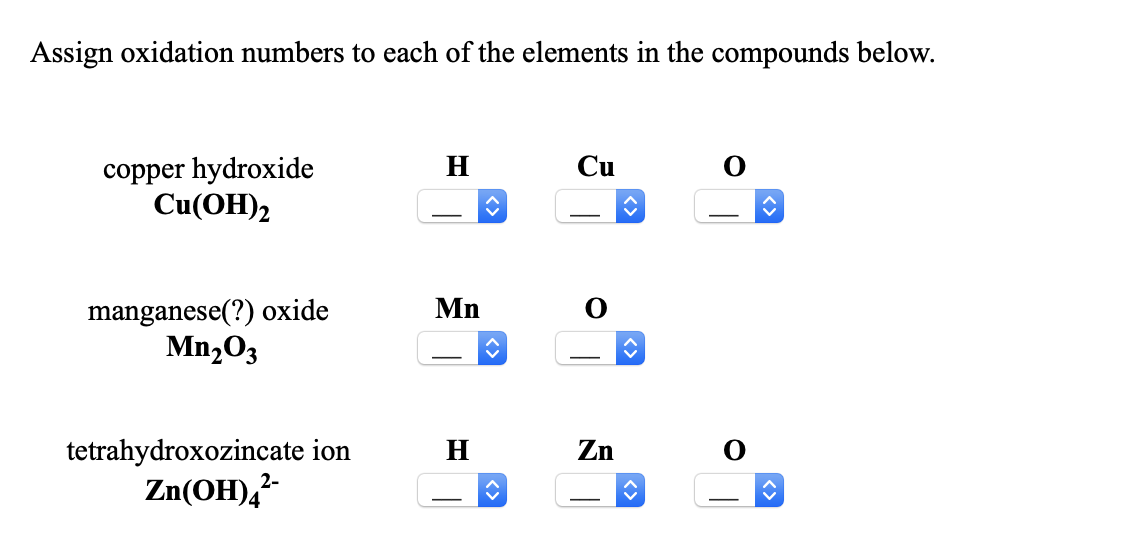
Solved Assign Oxidation Numbers To Each Of The Elements In | Chegg.com
