For lewis structure of co2, you will now have two oxygen atoms forming double bonds with a carbon atom. For oxygen atom ⇒ valence electrons of oxygen = 6

Resonance Structures For Co2 (Carbon Dioxide) - Youtube
6*2 = 12 total number of valence electrons = 16 find the central atom, which is usually the one with the highest bonding sites, is the carbon atom.

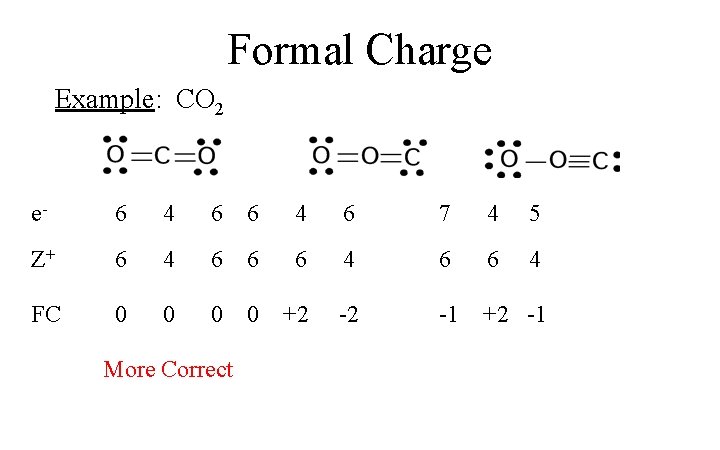
Co2 lewis structure with formal charges. Calculating the formal charge of co2 lewis structure by assuming the same electronegativity of every atom in the molecule like c and o. First compute for the formal charge of the atoms. There are two double bonds around carbon atom in the co 2.
Steps of drawing the lewis structure of co 2 are explained in detail in this tutorial. Begin your build by choosing a central atom and typing its element symbol. Here is the drawing of lewis structure of co with lone pairs and formal charges:
No lone pairs on carbon atom and each oxygen atom has two lone pairs on their valence shells. The formula we can use to calculate the formal charge, f.c. Lewis structure for co2 with formal charges at the top, the diamond comes in the list.
Carbon valence electron=4 oxygen valence electrons: Shape of co 2 is linear. Before answering, review the background material on how bond order affects bond length and the observed bond lengths in co2.
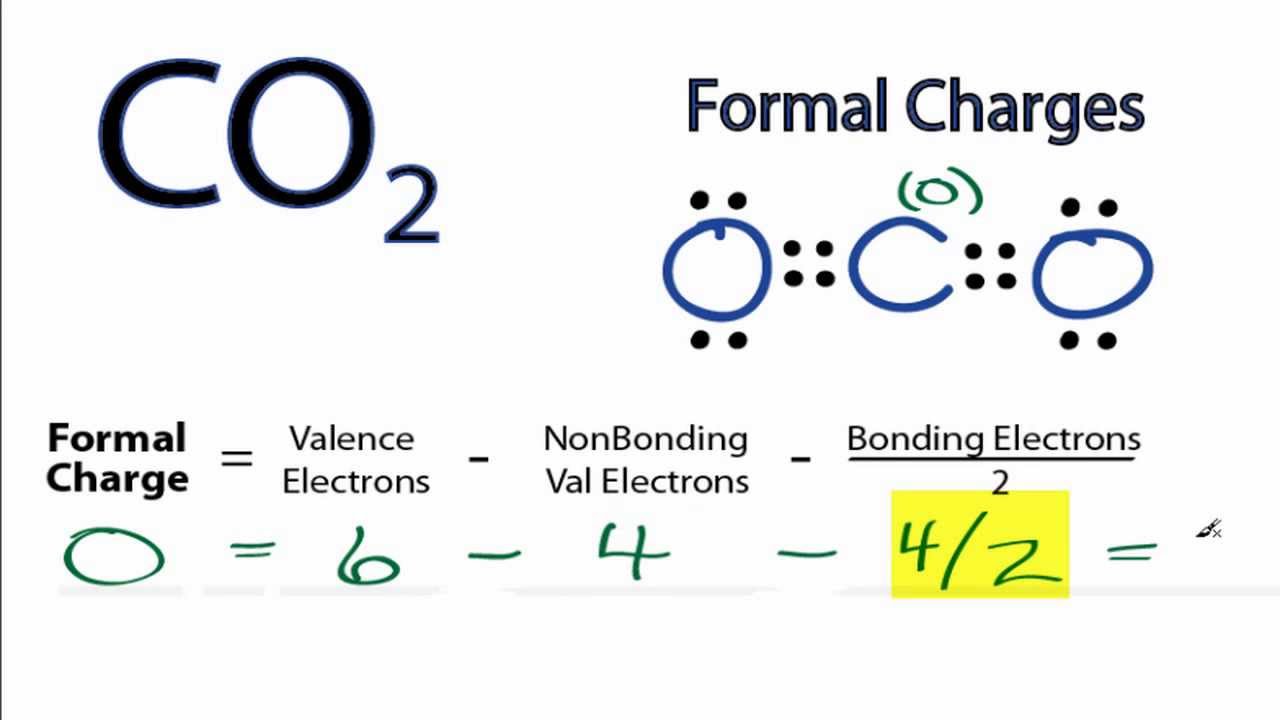
To find formal charges in a lewis structure, for each atom, you should count how many electrons it owns. Count all of its lone pair electrons, and half of its bonding electrons. 11.does the lewis structure with less formal charge predict the observed properties of co2 molecules better?
Let’s have co2 as an example. Caf 2 lewis structure has 16 valence electrons. The oxygen and carbon group families, which are the 16th and 14th groups in the periodic table, are both made up of oxygen and carbon atoms respectively.
Finally, this is our co2 lewis structure diagram. The total formal charge is 0. The carbon dioxide lewis structure and formal charge.(chem 1090 lewis 6a)
Let this picture to be our structure a. We will calculate the formal charge for the 5th step structure. For that, you need to remember the formula of formal charge;
For each of the hydrogens in methanol, we also get a formal charge of zero: To further understand the molecular geometry of co2, let us quickly go through its hybridization. The formal charge is a hypothetical concept and it has a particular formula to determine.
Valence electrons = 4 (as it is in group 14) How to draw a lewis structure? Draw four dots around it.
The difference between the atom's number of valence electrons and the number it owns is the formal charge. Two oxygenic armels establish covalent connections with the central carbon articles as a result. In the following calculation, the formal load will be calculated on the oxygen terminal spot of the lewis co2 points structure.
Now, you have come to the final step and here you have to check the formal charge on carbon atom (c) as well as each oxygen atom (o). So the formal charge on carbon is zero. The valence electrons, or outermost electrons of an atom, are released during chemical processes.
From this, we get no charge on the molecule. Let’s find out the formal charge for the individual atom of the co2 molecule. Let us calculate the caf 2 lewis structure valence electron.
The formal charge of an atom can be determined by the following formula: 6 steps on how to draw co2’s lewis structure calculate the total valence electrons found in a molecule. Co2 lewis dot structure by counting valence electrons on the carbon atom to calculate the valence electron of each atom in co2, look for its periodic group from the periodic table.
Carbon dioxide (co 2) lewis structure has two oxygen atoms and one carbon atom. Identifying formal charge on the atom. The fluorine atom and calcium atom both belong to the 2nd and 17th.
This will be the atom with the lowest electronegativity.
Lewis Dot Structures Developed By G N Lewis
Calculating Co2 Formal Charges: Calculating Formal Charges For Co2 - Youtube