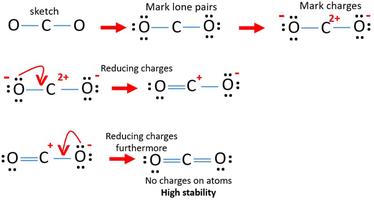
Resonance structures must also have the same amount of lone pairs. Given that co2 is a symmetrical molecule with two c=o bonds, i can’t imagine how there could be any reasonable resonance structures to resonate between!
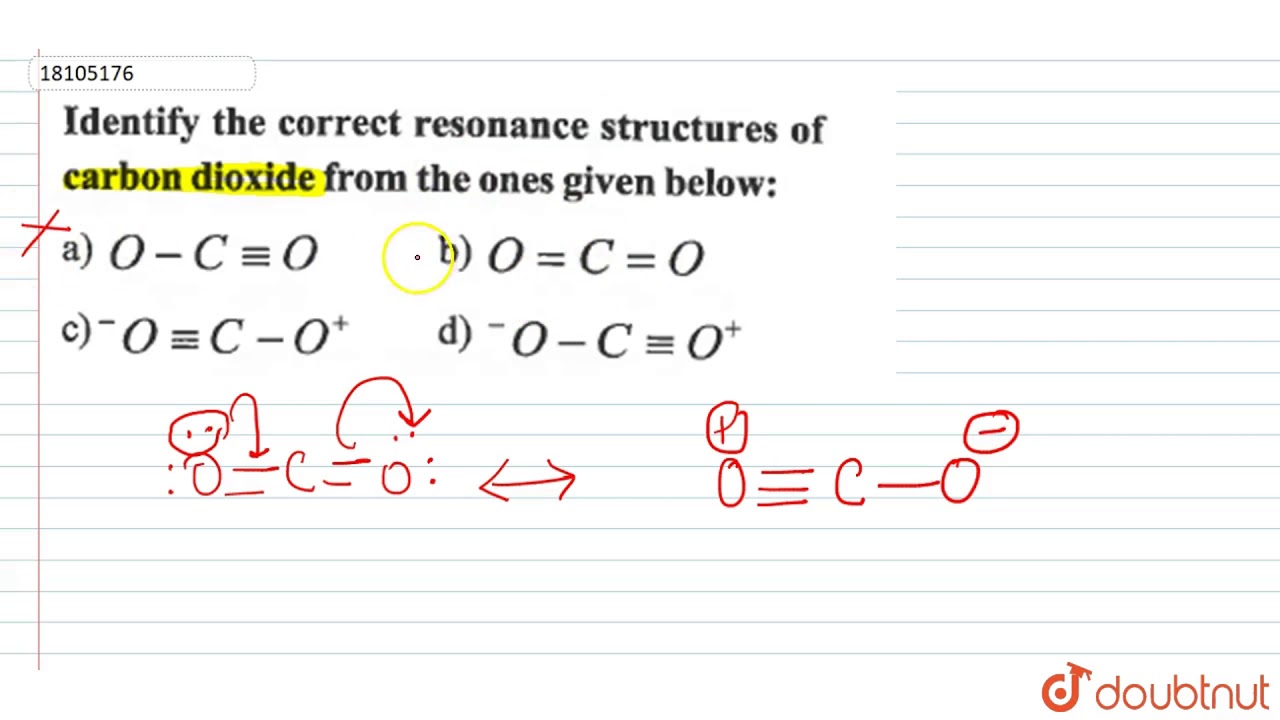
Identify The Correct Resonance Structures Of Carbon Dioxide From The One Given Below: - Youtube
After finishing the lewis structure of co.
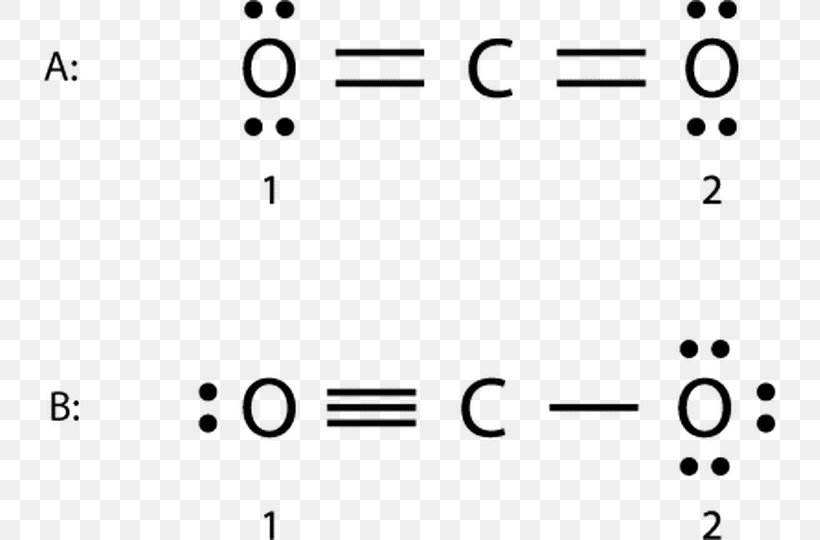
Co2 lewis structure resonance. When the electrons are in an. If a resonance hybrid of this polyatomic ion is drawn from the set of lewis structures provided above, the partial charge. Carbon dioxide, or co2 , has three resonance structures, out of which one is a major contributor.
Molecular geometry of co 2. However, there is an interesting resonance that is not discussed in the. The resonance structure accepts lone pairs of electrons, but the three lone pairs of electrons.
The co2 lewis structure shows that it has no lone pairs of electrons. Co 2 molecular geometry is based. Lewis dot structures can be drawn to visualize the electrons.
Since the overall formal charge is zero, the above lewis structure of co 2 is most appropriate, reliable, and stable in nature. For example could we get the. Co2 (carbon dioxide) is considered as a lewis acid due to the resonance structure of co2 which means it can accept a lone pair of electrons from lewis bases which.
Covalent bonds are created when an electron from one atom joins with an. A lewis structure is a way to draw out electrons and bonding by using dots. Do all lewis structures have resonance?
Feb 7, 2015 carbon dioxide, or co2, has three resonance structures, out of which one is a major contributor. In this tutorial, you can see how many resonance. This video discusses the resonance structu.
Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total. Now, in order to draw the lewis structure, we have to. Co2 or carbon dioxide is considered as acid or can even be called lewis acid.
= 4 + 6*3 + 2. Determine the central atom of the molecule. Lewis structure of carbonate ion is drawn in this tutorial step by step.
Co2 hybridization the electronic configuration of the carbon atom in its ground state is 1s22s22p2, and that of an oxygen atom is 1s22s2p4. Carbon has 4 valence electrons;. Co2 lewis structure (key points) linear geometry ( linear triatomic molecule) the bond angle is 180 °c.