Hope this helps :) answer link Is the molecule of ch4 is polar or nonpolar?
Solved Comparing Ch3Br (Polar) And | Chegg.com
In ch4 the sharing is equal.

Ch4 nonpolar or polar. Therefore ch4 is a nonpolar molecule. Ch4 is nonpolar.nonpolar means there is no polarization.the central atom is c and it has 4 valance electrons.ch4 makes 4 bonds and it has tetrahedral geometry.as a result,there is no polarity but if c makes less than 4 bonds there must be polarity. Ch4 is not very much dissolved in water.
13 mar methane or ch4 is a nonpolar molecule. Polarity results from an unequal sharing of valence electrons. You can also refer to the ch4 lewis structure first to understand the arrangement of atoms in the molecule along with its properties.
If more methane dissolved in water and if increase the temperature methane become less soluble. It has a molar mass of 16.043 g/mol and a density of 0.657 g/cm3 in solid at 25°c. But it isn't that shape, it is tetrahedral.
No ch4 or beh2 are nonpolar molecules. Th level 7 war base; Frequently asked questions (faqs) some of the frequently asked questions are given below:
Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. The bond angle is 109.5 degrees,and there are 8 valence electrons. Properties of ch4 ch4 is a colorless, odorless gas that is the most basic of all hydrocarbon compounds.
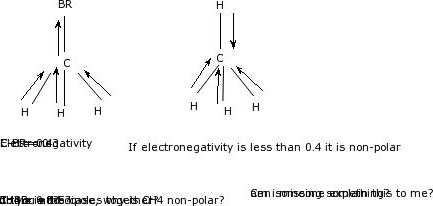
Ch4 contains nonpolar covalent bonds because the electronegativity difference between hydrogen (2.20) and carbon (2.55) is lower than.5. So, you can say that, ch4 is nonpolar but h2o is polar molecules. Ch4 is nonpolar because all of the nonpolar covalent bonds are spaced within a tetrahedral structure around the molecule.
We can consider that methane is nonpolar based on these solubility factors. Hence the ch4 molecule is a nonpolar molecule. To find out the reason why the molecule is nonpolar, let’s go through the factors that help us determine the polarity of the molecules.
Co2 polar mı apolar mı ch4 polar mı apolar mı is ch4 polar or nonpolar? This distributes electron charge equally around the central carbon atom. While there may be a difference in electronegativity between the.
Similarly, for different other gases, such as nitrogen helium oxygen if temperature increase, they become less soluble. Polar in chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Exposure to methane can be dangerous.
Ch4 is non polar.it is a tetrahedral compound. It has no lone pairs.so it.

Is Ch4 Polar Or Nonpolar? (Special Concepts) - Chemwhite.com