Find the decay constant for this process. Refer to question 9 above:
The binding energy is [a] 1 s 2.

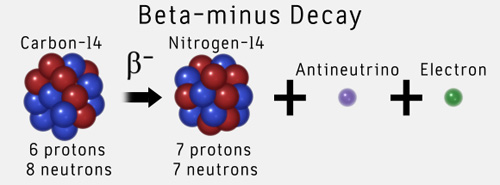
C14 decays by beta emission. 14 c decays by a process called beta decay. The radioisotope c 1 4 decays spontaneously by β − e m i s s i o n. This in turn means the element # has increased by one, becoming nitrogen.
1 a beta emission decay results in the release of 0 −1β − 1 0 β. Neutron mass = 1.008665 amu; For example, beta decay of a neutron transforms it into a proton by the emission of an electron accompanied by an antineutrino;
Expert answer previous question next question Carbon 14, 146 c, has six protons in its nucleus and is formed in our atmosphere by cosmic ray bombardment of nitrogen 14, 147 n. Find the time it takes for activity of bone sample to be reduced by.
1kg =6.022 x 10 26 amu; ^14c decays into ^14 n via what type of decay? Example 2 carbon 14 beta decay.
(proton mass = 1.007825 amu; Gamma by signing up, you'll get thousands. View the full answer transcribed image text:
During this process, an atom of 14 c decays into an atom of 14 n, during which one of the neutrons in the carbon atom becomes a proton. Beta decay, which can be thought of as the conversion of a neutron into a proton and a β particle, is observed in nuclides with a large n:p ratio. Just a reminder, from special relativity difference in.
Such nuclei lie above the band of stability. The beta particle (electron) emitted is from the atomic nucleus and is not one of the electrons surrounding the nucleus. If an artifact is found to be 34,380 years old, what fraction of the original.
A reducing the size of certain tumors generating radiation for medical imaging c producing energy in nuclear power plants determining the age of buried organic materials. Carbon 14 is unstable and undergoes radioactive decay by emitting a beta particle (an electron) and an antineutrino (zero mass, zero charge) and becoming nitrogen (seven protons): It constantly disappears by the process of negative beta emission, in which a neutron in the nucleus disintegrates into a proton, an antineutrino, and an electron.
And 1 j = 1kg m 2). C14 decays into the stable isotope c 12 with a half life of 5730 yrs ( 1.8*10 11 11 s). The c14 decays into nitrogen 14 whereby a so called extra neutron changes into a proton and an electron that takes its place in the outer 'orbital'.
Reason β − e m i s s i o n leads to conversion of a neutron to proton, resulting in the decrease in n / p ratio. Refer to question 9 above:
What Is The Beta Decay Equations? + Example

Alpha Decay Alpha Particles Consist Of Two Protons Plus Two Neutrons. - Ppt Video Online Download
