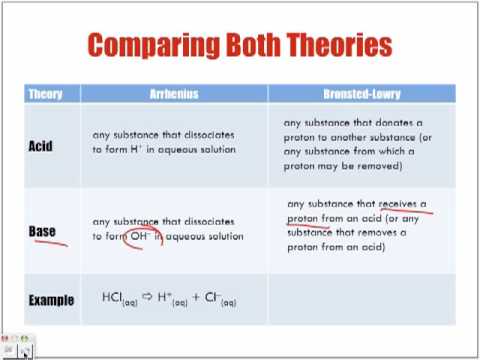
The only difference is that the solution does not have to be water. The acids and bases are represented in this bronsted lowry theory in the way they react with each other, which makes for greater generality.
Acid-Base Definitions | Ck-12 Foundation
In the example of ammonia dissolving in water.
Bronsted lowry lewis acid. About us become a tutor blog download app. Filo instant ask button for chrome. When a proton ( h+) accepts electrons donated from a lewis base (like oh− ), it.
A base is a proton (hydrogen ion) acceptor. Bronsted put this theory forward in 1923. At the same time, thomas lowry independently presented the same theory.
A lewis acid needs to have the ability to accept a lone pair of electrons, so it needs an empty orbital to. The relationship between the bronsted. Links to related reading in textbook (mcmurry, organic chemistry, 4th.
Ez a cikk az egyes. Therefore, this definition is known as bronsted. This definition is expressed in terms of.
A bronsted lowry acid needs to have a proton (h+) it can donate. Bu makale, her birinin tanımını, ayrıca asit. Lewis states that an acid is a substance that accepts a pair of electrons to form a new bond.
In contrast, arrhenius says that an acid is a substance that increases the concentration of. An acid is a proton donor, and a base is a proton acceptor.

Conjugate Acid Base Pairs, Arrhenius, Bronsted Lowry And Lewis Definition - Chemistry - Youtube

/chapter3/pages19and20/page19and20_files/lewisbronsted.png)