Mg (s) = 0 charge. The charges and sizes of the ions in an ionic compound affect the strength of the electrostatic interaction between the ions and thus the strength of the lattice energy of the ionic compound.

Chemistry Chapter 3: Compounds And Molecules Flashcards | Quizlet
What type of substance is kbr?
Kbr compound charges. You can use parenthesis () or brackets []. Yes, there is an ionic bond present in between the k and br which is not form by sharing of electrons and so it is an ionic compound u can also identify it as an ionic bond as potassium is a metal and bromine is a non metal…. Compound states [like (s) (aq) or (g)] are not required.
Kbr is an ionic compound ionic compounds have. Overall, it is a neutral compound since one unit of positive charge in the complex is balanced by one unit of negative charge,. K (s) = 0 charge.
Draw a bohr diagram to show the electron transfer that occurs when magnesium and. Learn about the potassium bromide formula and kbr compound. The oxidation number of any atom in its elemental form is 0.
How does the sum of the charges on the positive ions compare to the sum of the charges on the negative ions in ionic compounds? The sum of oxidation numbers in a neutral compound is 0. Examples potassium + bromine = potassium bromide k + br = brk k + br = brk1122334 k + br = k + br k + br = k2br k + br = k2br2 k + br = k2br3 k + br = k3br k + br = k7br (cooh)2.2h2o + naoh = nacoo + h2o h2o2 + n2o4 = n2 + h2o + o2
(4) kbr → k++br− k b r → k + + b r −. The resulting atom charges then represent the oxidation state for each atom. Names and formulas for ionic compounds 1.
The compound mgs and mgcl2 m g c l 2 has +2 charge, but sulfur has a large size in comparison to chlorine, therefore mgcl2 m. Naf kbr mgf2 mgf2 mgo; Here the magnitude of charge is 1.
Waltrip indoor training center (waltrip center), 6100 main street, houston, tx 77005 Kbr is an ionic compound ionic compounds have relatively high mp that depend on. [molar mass of 119 g/mol kbr] * 3 mol kbr = 357 grams for 3 moles kbr what does kbr.
Calculate the sum of the ionic charges in the compound al 2o 3. Kbr(aq) + agno 3 (aq) → agbr(s) + kno 3 (aq) the reaction of bromide in its aqueous form with metal halides produces complex. How many grams are in 3 moles of kbr?
Based on ion charges and relative ion sizes, rank these ionic compounds by their expected melting points from highest to lowest. Melting point depends on the following factors: 2 kbr(aq) + cubr 2 (aq) → k 2 [cubr 4](aq) health hazards.
M gbr = m g+2br−1 2 ( balanced compound) kbr = k+1br−1 ( balanced compound) k(s+m gbr2 = kbr +m g(s) there are two br in the reactants but. The charges and sizes of the ions in an ionic compound affect the strength of the electrostatic attraction holding that compound together. The sum of the oxidation numbers in a monatomic ion is equal to the overall charge of that ion.
For example, the reaction of potassium bromide and copper(ii) bromide produces a complex compound. Kbr is a type of salt. Naf kbr mgf2 mgf2 mgo
The chemical equation for the same is given as;
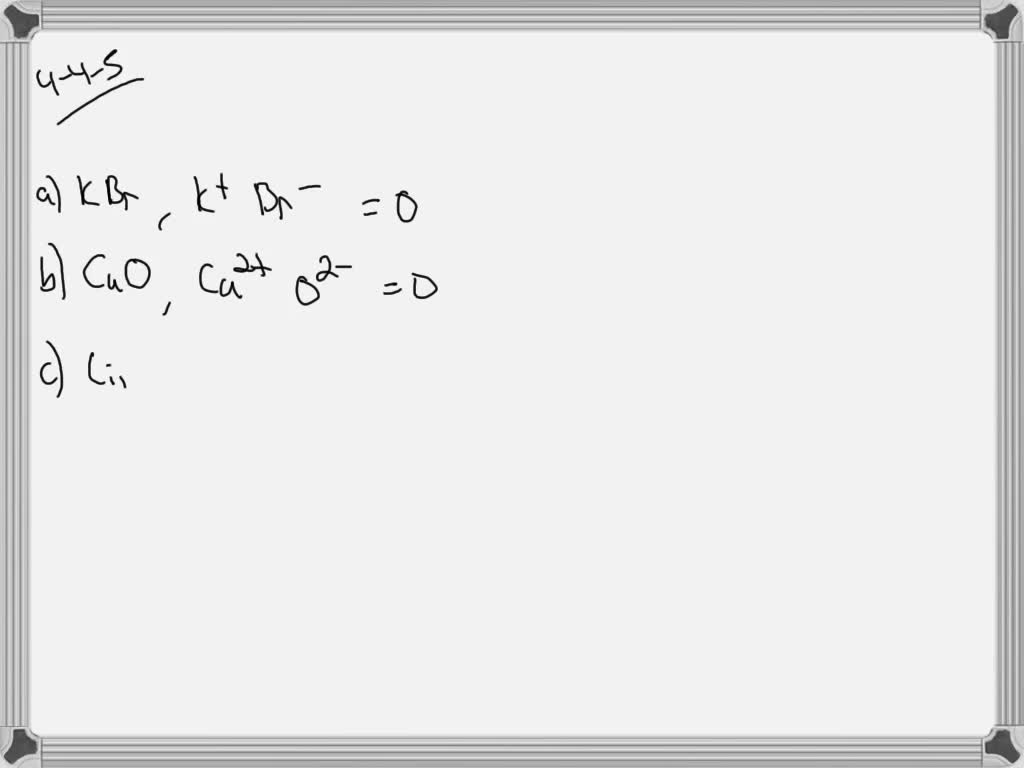
Solved:for Each Of These Compounds, Show That The Charges On The Ions Add Up To Zero. A. Kbr B. Cao C . Li_2 O D . Cacl_2 E. Alcl_ _3
