They will differ in the number of neutrons held by their respective nuclei. Isotope notation is particularly important in nuclear chemistry, because if you're doing fission, fusion,.

Makethebrainhappy: Isotope Notation
The table shows the approximate occurrence rates of naturally occurring hydrogen and oxygen:
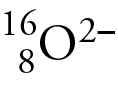
Isotope notation definition. A stable isotope is an atom that, although it has either a lack or an excess of neutrons, won ’ t decay or disintegrate. The vast majority of water molecules consist of two 1 h and one 16 o atom. You can find many examples of stable isotopes among the elements with a low atomic number.
The element is defined by the number of protons in the nucleus but the number of neutrons can vary. Isotope examples carbon 12 and carbon 14 are both isotopes of carbon, one with 6 neutrons and one with 8 neutrons (both with 6 protons). Rsamp and rref are the absolute isotope ratios of the sample and reference material respectively.
Where 6 ref is the isotope ratio of the sample expressed in delta units relative to the reference material. Isotopes are notated in multiple ways. Isotopes of an element share similar chemical properties, but have different nuclear properties.
Isotopy of loops, a triple of maps with certain properties. An isotope is a variation of an element that possesses the same atomic number but a different mass number. Isotope notation isotope notation subscripts and superscripts can be added to an element’s symbol to specify a particular isotope of the element and provide other important information.
Isotopes are variations of atoms classified as being the same element. An isotope is described as a variation of an element that possesses a similar atomic number but with a different mass number. Isotopes and the delta notation all water molecules consist of two hydrogen atoms and one oxygen atom, but there are different stable isotopes of hydrogen and oxygen.
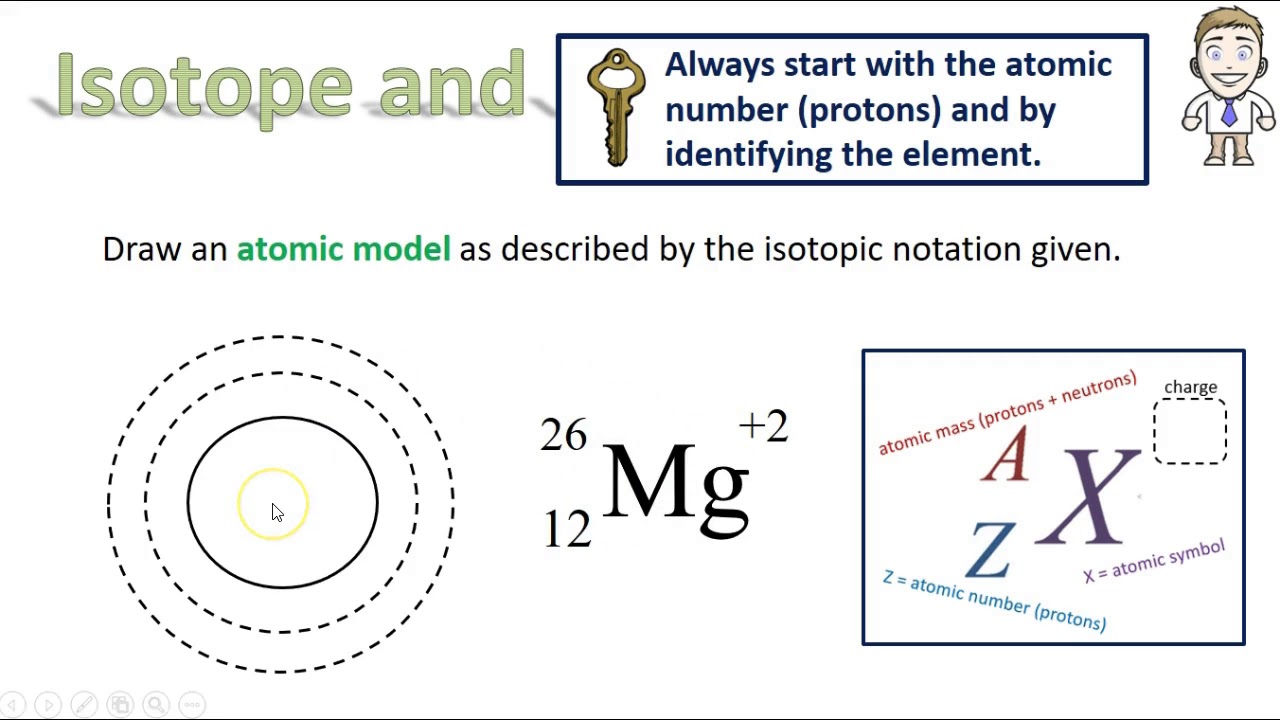
Moreover, they will vary in the number of neutrons held by their respective nuclei. Isotopes are the atoms of an element with different numbers of neutrons. You put the atomic number, mass number, and net charge around the chemical element symbol.
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. All isotopes of an element have the same atomic number and number of protons, but they have different atomic masses from each other. They have the same proton number, but different mass numbers.
While all isotopes of a given element have almost the same chemical properties, they have differ… 1 + (<513c%o source.10~3) which can be simplified to equation 7.5. Definition of isotopes isotopes are atoms of the same element which contains the same number of protons but different number of neutrons.
When abbreviated, the element's symbol is used, prefixed by a superscripted mass number, above a subscripted atomic number. A group of isotopes with any element will always contain a similar number of electrons and protons. 289 rows isotope, one of two or more species of atoms of a chemical element with the.
Isotopes are forms of an element that have different numbers of neutrons. The term isotope was introduced by the british chemist frederick soddy in 1913, as recommended by margaret todd. Carbon 12 and carbon 13.
1 2 3 glossary move. Isotope names generally include the name of the element followed by the atomic mass. When employing formal scientific isotope notation, an isotope is usually expressed in longhand as the name of the chemical element, followed immediately by a dash, and then the mass number (a), i.e.
Multiplying by 1,000 converts the value to parts per thousand (%o), or. A group of isotopes of any element will always have the same number of protons and electrons. When abbreviated, the element's symbol is used, prefixed by a superscripted mass number, above a subscripted atomic number.
Copper 63 and copper 65. When employing formal scientific isotope notation, an isotope is usually expressed in longhand as the name of the chemical element, followed immediately by a dash, and then the mass number (a), i.e. [noun] any of two or more species of atoms of a chemical element with the same atomic number and nearly identical chemical behavior but with differing atomic mass or mass number and different physical properties.
Isotopes can also be defined in standard, or aze, notation where a is the mass number, z is the atomic number, and e is the element symbol.