If it is larger than this value, then the battery may have problems. The only way for this to make sense is if half the atoms of oxygen are positive and half are negative bring a positive charge of 4 and negative charge.

Calculate The Formal Charge On Phosphorus Atom In Po4 3- - Brainly.in
During charging and discharging, there will be some voltage changes in lifepo4 batteries.
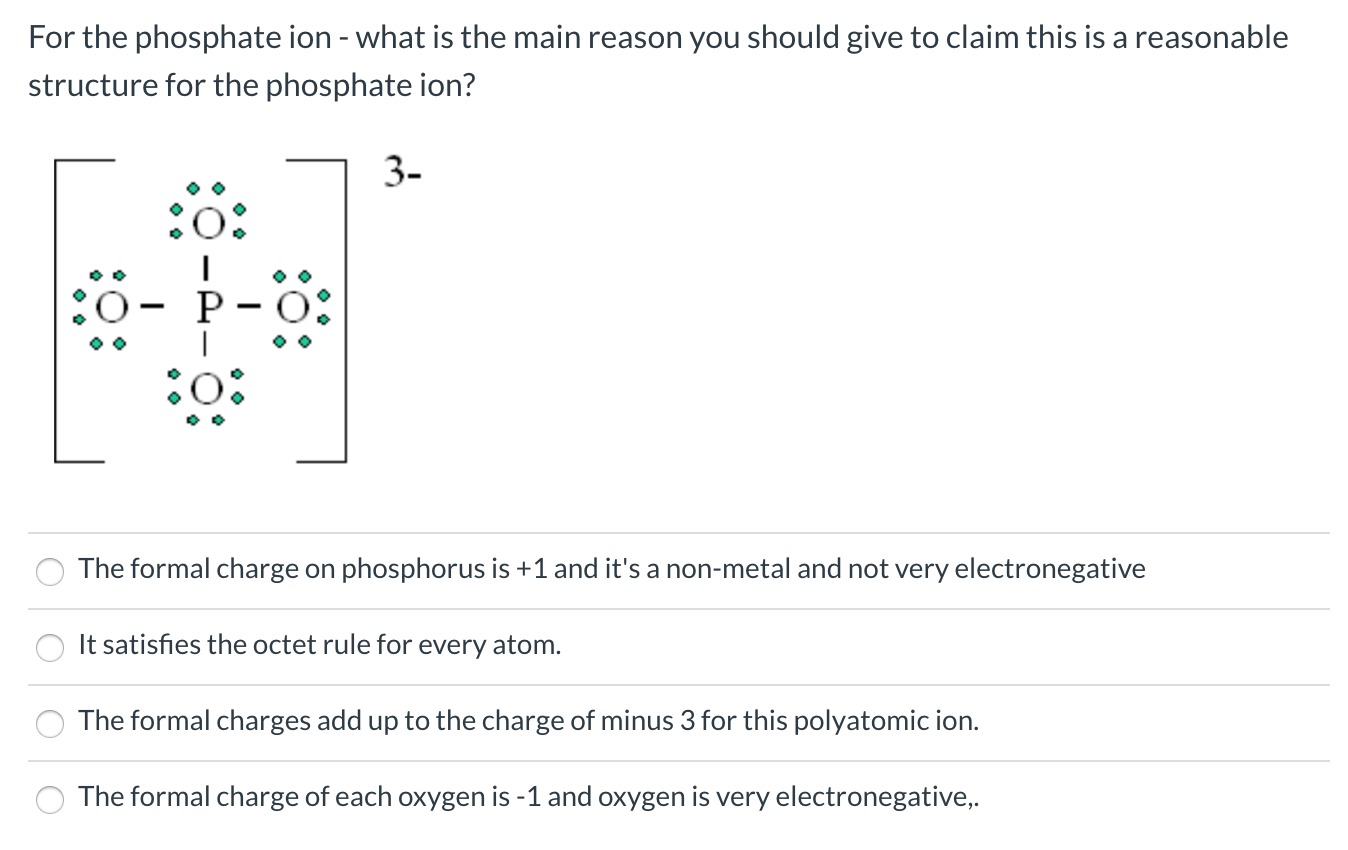
Charge of p in po4. 2 ( x) + 7 ( − 2) = − 4 2 x − 14 = − 4 2 x = 10 ⇒ x = + 5. Stage 2, constant voltage, begins when the voltage reaches the voltage limit (14.7v for fast charging sla batteries, 14.4v for most others). Add your answer and earn points.
To calculate the formal charge, the following formula is used. O belongs to group 6, hence has 6×4= 24 valence electrons (4 atoms of o). So, the correct answer is “option a”.
What is the name of the. Most agm battery chargers are within that range and they would be compatible with canbat lithium batteries. The most common charges are based on maximum stability for the atom.
Many hach spectophotometers and colorimeters give the option to change the chemical form of the reported value. In order to fully charge a 12v lifepo4 battery, a charger with a voltage of 14v to 14.6v is required. I am studying chemistry in high school and i am studying my ions (because i failed too in the beginning, oops) and i have a question on this reasoning:
During this stage, the current draw gradually decreases as the topping charge of the. By the loss of h +.). P belongs to group 5, hence has 5 valence electrons.
So the oxidation number of p is calculated as follows: Po4 molecular weight molar mass of po4 = 94.971361 g/mol convert grams po4 to moles or moles po4 to grams molecular weight calculation: If you have a charger with a lower voltage, it may still charge the battery, but it won’t charge it to 100%.
Although noble gas atoms almost always carry a charge of zero, these elements do form compounds, which means they can gain or lose electrons and carry a charge. Sponsored by siriusxm amar nath garg Phosphorous is a group 15 element.
Did you mean to find the molecular weight of one of these similar formulas? We'll draw bonds between the oxygen and the phosphorus. However, other charges are possible.
Answer 1.0 /5 5 brainly user explanation: Therefore, the oxidation number of phosphorus in p o 4 3 −, p 4 o 10 and p 2 o 7 4 − is +5.

Calculating Po43- Formal Charges: Formal Charges For The Phosphate Ion - Youtube

Calculating Po43- Formal Charges: Formal Charges For The Phosphate Ion - Youtube