Calcium additives, however, may cause a loss in power and/or cycle life. Calcium sulfide is the chemical compound with the formula ca s.
Solved Determine The Charge On Each Ion In The Following | Chegg.com
Remember that electrons are negative charges, and protons are positive charges.

Calcium sulfide charge. Oldhamite is the name for mineralogical form of cas. How old was tom felton when he lost his virginity? Post date 5 oraciones con el verbo take en pasado;
What is the correct symbol and charge when calcium forms an ion? Is calcium and sulfur ionic or covalent? Two crystalline forms are known, the hemihydrate and the tetrahydrate, respectively caso 3 ·½ (h 2 o) and caso 3 ·4 (h 2 o).
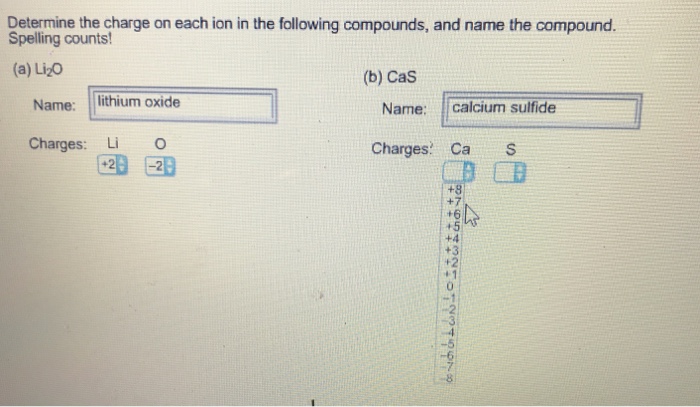
Calcium sulfide formula with charges. How do you find the charge of a calcium ion? The calcium possesses a +2 charge while the sulfur anion possesses a −2 charge and so one calcium cation neutralizes one sulfur anion.
What is the charge of calcium sulfide? However, the high charge density of ca 2+ contributes to strong electrostatic interaction between divalent ca 2+ and hosting lattice, leading to sluggish kinetics and poor rate performance. A step by step explanation of how to draw the cas lewis dot structure.for cas we have an ionic compound and we need to take that into account when we draw th.
In this video we'll write the correct formula for cas (calcium sulfide). This white material crystallizes in cubes like rock salt. [2] all forms are white solids.
What charges do ions form? Calcium sulfite, or calcium sulphite, is a chemical compound, the calcium salt of sulfite with the formula caso 3 ·x (h 2 o). Nickel hydroxide active material can be formulated for specific applications.
For operation at 65 °c, some manufacturers add calcium hydroxide, calcium fluoride, calcium sulfide, calcium oxide, or yttrium oxide to the paste to inhibit early oxygen evolution on charge. This formula indicates that this compound is made up of twice as many sodium ions as sulfide ions. Your answer should include a discussion of subatomic particles.
Calcium sulfide sulphide, cas or sca, is ionic because it is made from a metal and non metal.a calcium atom loses two electrons to become 2 chargeda sulfu. To write the formula for cas we’ll use the periodic table and follow some simple rules. Nature of oxide and sulphide inclusions present at 1550 °c as functions of the total calcium and oxygen content in a steel grade with 1.5% mn, 0.2% si, 0.04% al and 0.005% s.
Calcium is a chemical element found in nature. An ion is an atom of a chemical element that has an unequal number of electrons compared to protons. Keys for writing formulas for binary.
Who is the longest reigning wwe champion of all time? The difficulty arises from several factors. Ron kovic speech, 1976 democratic convention
Positively charged ions are called cations negatively charged ions anions. Rent a villa or vacation home! +2 charge calcium sulfide is a molecule with the chemical formula cas.
From an industrial point of view, the adjustment of a ca treatment may be problematic. Because the net ionic charge of a compound is zero, only one of each atom is needed to create calcium sulfide, which will be cas. La roche posay anthelios xl ultra light on calcium sulfide formula with charges.
The charge of the metal ion is determined from the formula of the compound and the charge of the anion. Calcium sulfide formula with charges calcium sulfide decomposes upon contact with water, including moist air, giving a mixture of ca (sh)2, ca (oh)2, and ca (sh) (oh). In the case of the calcium ion, we have a calcium element with a positive charge of 2.
Ion any atom or group of atoms that bears one or more positive or negative electrical charges.
Calcium Sulfide | Cas - Pubchem

How To Write The Formula For Cas (Calcium Sulfide) - Youtube