It is the average acceleration, which in the case of u.a.r.m. Acceleration (a) is the rate of change of velocity (v) with respect to time (t) or a = dv/dt the formula for average acceleration during an interval of time is average acceleration = (final.
Acceleration-Time Graphs - Ib Physics - Youtube
Click ‘start quiz’ to begin!

Acceleration over time graph. She has over 10 years of experience developing stem curriculum and teaching physics, engineering,. Learn how to calculate speed, velocity and acceleration. In this case, value of velocity will be maximum at the last point of time.
Jerk is most commonly denoted by the symbol j and expressed in m/s 3 ( si units) or standard gravities per second (. It is a vector quantity (having both magnitude and direction). By looking a speed vs.
Acceleration on a position vs. The graphical representation of acceleration over time can be derived through the graph of an object's position over time. Acceleration using a velocity time graph example problems with solutions example 1.
Find out how to use distance time graphs and velocity time graphs with bbc bitesize gcse physics. In the above graph, the velocity corresponding to time = 5 s, is the highest. Or metre per second per second, as the velocity in metres per second changes by the acceleration value, every second.
Acceleration is defined as, δ a = δ v δ t by multiplying both sides of the equation by the change in time δt, we get δ v = a δ t substituting the values in the above equation,. In physics, jerk or jolt is the rate at which an object's acceleration changes with respect to time. Test your knowledge on graphs put your understanding of this concept to test by answering a few mcqs.
Here's what an acceleration vs time graph might look like for a moving particle: What is the acceleration of the van? More realistic motion maps in the real world, acceleration is frequently not constant.
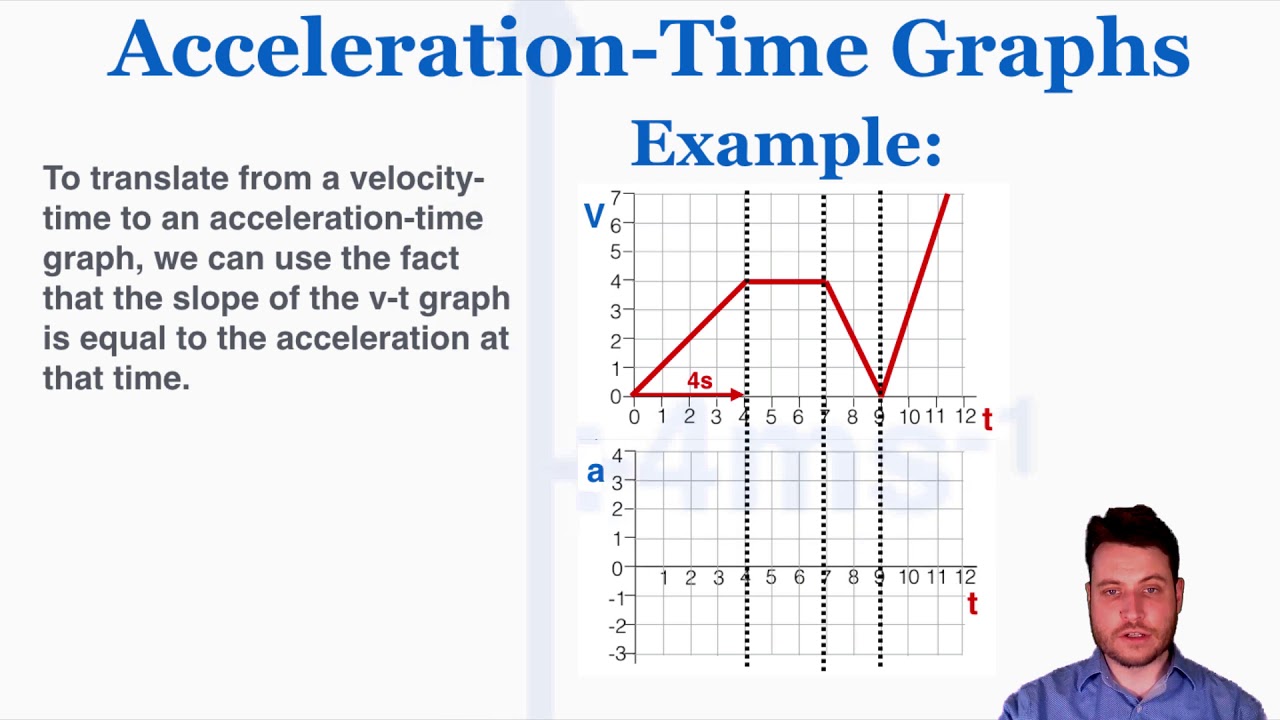
A = f / m, where: The graph below shows a constant acceleration of 4 m/s 2 for a time of 9 s. A is the acceleration, v_i and v_f are respectively the initial and final velocities, δt is the acceleration time, δd is the distance traveled during acceleration, f is the net force acting on an object that accelerates, m is the mass of this object.
L t −2.the si unit of acceleration is the metre per second squared (m s −2); The units of acceleration are m/s/s or m/s2. Acceleration on a speed vs.
Now you know how to calculate acceleration! Acceleration can be calculated by dividing the change in velocity (measured in metres per second) by the time taken for the change (in seconds). An object moving in a circular motion—such as a satellite orbiting the earth—is accelerating due.
The acceleration at time 0 is 0, then becomes positive, and finally, at 9 seconds, it returns back to 0. Often, to show how the acceleration of a particle changes over time, an acceleration vs time graph is used. Learning objectives distinguish the difference between how to plot a velocity graph and how to plot an acceleration graph key takeaways key points acceleration is the rate at which the velocity of a body changes with time.
Is the same as the instantaneous acceleration. Acceleration has the dimensions of velocity (l/t) divided by time, i.e.
It is the relationship between heat and temperature change. Heat capacity is a measure of the amount of heat needed to raise the temperature of a substance one degree.

Solved Why Is It Important To Determine The Heat Capacity Of | Chegg.com
Water has a high specific heat, meaning it takes.

Why is heat capacity important. This substance size corresponds to the amount of heat needed to heat a certain amount of a substance by one. It is often used as a measure of productive efficiency. In connection with the specific heat capacity, the term capacity is intended to describe the ability of a substance to absorb heat without any noticeable change in.
Water has an especially high heat capacity at 4.18 j/g*c, which means it takes more heat to warm a gram of water. It is a thermodynamic property of the substance. This is why, throughout the course of a warm summer day, the water in.
The specific heat capacity indicates the ability of a substance to store heat. Heat capacity is defined as the amount of heat required to raise the temperature of a given object by 1 kelvin (si unit of heat capacity j k−1). On the other hand, the amount of heat.
Heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). In si specifications, we recognize specific heat through symbol 'c' and the unit is joule per kelvin.
The high specific heat capacity of water, 1 cal/g x degree c is what enables the oceans to absorb energy and assist in stabilizing our planets livable conditions. This is obviously important because organisms can only live in a certain. What is an example of high heat.
Water is the substance with the second highest known specific. The waters heat capacity (as well as its heat vaporation) allows it to moderate earths climate. Capacity utilisation is an important concept:
As the water evaporates, energy is taken up by the process, cooling the environment where the evaporation. A high specific heat of a substance means that a large amount of heat is required to raise the temperature of the substance. It is also what enables ‘warm.
Heat capacity for a given matter depends. Why is high heat of vaporization important to living organisms? Water covers around 70% of the earth’s surface and its high specific heat plays a very important role as it is able to absorb a lot of heat without a significant rise in the.
The trigonometric ratios sin, cos, and tan for the angle a are as follows. Practice your math skills and.

Solved Use Trigonometric Identities To Write Cos X In Terms | Chegg.com
And we want to know d (the distance down).

Cos in terms of sin. If the acute angle θ is given,. Lets suppose we have triangle. The given problem can be solved applying the trigonometric identity sin2θ+cos2θ= 1 sin 2 θ + cos 2 θ = 1, a fairly typical trigonometric identity.
Tan (x) = sin (x)/cos (x) therefore, all trigonometric ratios can be expressed in terms of sin and cos. The complementary angle equals the given angle subtracted. Cos is a type of lettuce.
1 to express cosθ cos θ. Using pythagorean identity sin2x +cos2x = 1, so cos2x = 1 − sin2x cosx = ± √1 − sin2x sinx +. The birth of the terms ‘cosine’ and ‘tangent’ was much later.
Answered • 01/06/16 tutor 4.8 (24) patient and. Another way to prove it is to draw a right angle triangle with a hypotenuse of unit length. What is cos equal to?
The cable's length is 30 m. So the identity can be rewritten in terms of sin and cos. Dariel barroso's answer is correct (to check it, the addition formula can be used).
Let a be the length of the side opposite angle a, b the length of the side adjacent to angle a and h be the length of the hypotenuse. In trigonometry, sin cos and tan values are the primary functions we consider while solving trigonometric problems. These trigonometry values are used to measure the angles and sides.
Start with the pythagorean identity (sinx)^2 +. Please see two possibilities below and another in a separate answer. The angle the cable makes with the seabed is 39°.
For example, you may have some sine terms in an expression that you want to express in terms of cosine, so that all the functions match, making it easier to solve an equation. Sin is a religious concept; Trigonometric ratios (sin, cos, tan, cot, sec and cosec) these six trigonometric ratios form the base of trigonometry.
The sine of an angle is equal to the ratio of the opposite side to the hypotenuse whereas the cosine of an. Sin and cos are basic trigonometric functions along with tan function, in trigonometry. Relations between cosine, sine and exponential functions (45) (46) (47) from these relations and the properties of exponential multiplication you can painlessly prove all sorts of trigonometric.
Sin cos tan formulas the important sin cos tan formulas (with respect to the above figure) are: Definition of cosine the cosine of an angle is defined as the sine of the complementary angle. Then there are only two.
Now, if you want to express sinx in terms of cosx for some number x, that’s another question. The cosine function comes to light from the need to calculate the sine of the complementary angle. In a right triangle abc, solution:
If the slope of the curve y= b−xax at the point (1,1) is 2, then the values of a and b are respectively a 1,−2 b −1,2 c 1,2 d none of these hard solution verified by toppr correct. Mathematics, 21.06.2019 22:00 `if you have a set of parallel.

Differentiation From First Principles - Slope Of The Curve F(X) = X² - Youtube
In this case, we can take the derivative of y with respect to x, and plug in the desired value for x.

Slope of the curve at a point. In this video, i discuss one of the first few concepts that are learned in any calculus course: You can take whichever one you want, or even average the slopes on each side if. The slope of a function, f, at a point x = (x, f(x)) is given by m = f '(x) = f '(x) is called the derivative of f with respect to x.
Find an equation of the tangent line to the curve at \ (. The point where the curve and the tangent meet is called the point of tangency. Application of slope of tangent is temperature change at a particular time, velocity of a falling object at a particular time, current.
The slope of a curve at a point. 1 show answers another question on mathematics. The equation of the curve is a x 2=y+5 b y 2=x−5 c y 2=x+5.
Recall that the slope of the curve at a point p (x,y) is dy dx. To find the slope at a point of our function, we need to find its derivative first. This is a separable variable type.
The slope of a tangent line can be found by finding the derivative of the curve f (x and finding the value of the derivative at the point where the tangent line and the curve meet. So the slope of each normal line is the opposite reciprocal of the slope of the. The slope of a curve is a slope of a tangent line for a curve at one point.
If a curve passes through the point (1,−2) and has slope of the tangent at any point (x,y) on it as x2−2y x, then the curve also passes through the point : D y d x = d d x ( 2 x 3 8 x 2 + 1) d y d x = 6 x 2 16 x. To find the slope of a curve at a given point, we simply differentiate the equation of the curve and find.
Other names for f '(x): Using the product rule (and the chain rule within this product rule application), we have. Therefore, by what has been given, we have, dy dx = 2y.
One way of finding the slope at a given point is by finding the derivative. The slope of a curve at any point is the reciprocal of twice the ordinate at the point and it passes through the point (4,3). Each normal line is perpendicular to the tangent line drawn at the point where the normal meets the curve.
A tangent is a straight line that touches a curve at a single point and does not cross through it. Find the slope of the curve \ ( y=x^ 3+3 \) at the point \ ( p (2,11) \) by finding the limiting value of the slope of the secants through p. ⇒ dy y = 2dx.
I picked another point q to get the secant. A (3,0) b (−1,2) c (−√2,1) d (√3,0). Viewed 24k times 0 find the slope of the curve y = x 2 − 4 x − 5 at the point p ( 3, − 8) by finding the limit of the secant slopes through point p.
This will yield the equation of the tangent line to the function eqf(x)/eq at the given. 7b slope of curve 4 definition: For each point, you will have a slope to the right of the point and a slope to the left of the point.
The slope of a curve at. Curve at a particular point. To find the equation of a line, we need the slope of the line and a point on the line.
Slope of a curve the slope of a curve at a point p is de ned to be the slope of the tangent line to the curve at p.
Also, is oxygen a metal or. It belongs to the oxygen family.
Where Is Sulfur In The Reactivity Series? - Quora
Sodium metal is soft whereas sodium chloride is very hard.
Is sulfer a metal. On the periodic table, sulphur is in group 16/via, in the third period. Sulfur (in british english, sulphur) is a chemical element with the symbol s and atomic number 16. All elements belonging to group 6a are collectively called chalcogens.
Sulfur is a chemical element with an atomic number of 16 and an atomic symbol of s. Similarly one may ask, is sulfur a metal or non metal? It is a poor conductor of heat and electricity, because electrons are not free to move.
The sulfur atom holds onto electrons too tightly to be a normal metal. Most reactive to least reactive metals: Sulfur is abundant, multivalent, and nonmetallic.
Arrange the following elements in order of increasing ionization energy: Sulfur can be represented in the periodic table as follows: It is a poor conductor of heat and electricity, because electrons are not free to move.
The chemical symbol for sulfur is s. Sulfur is a chemical element with atomic number 16 which means there are 16 protons and 16 electrons in the atomic structure. What is the only metal that is in liquid form at room temperature?
On the periodic table, sulfur is in group 16/via, in the third period. Pure sulfur is a tasteless, odourless, brittle solid that is pale yellow in colour, a poor conductor of electricity, and insoluble in water. It does not have luster, it cannot conduct electricity, and it is brittle.
The group is called the oxygen group or. Most reactive to least reactive metal cations: How does sulfur react with metal?
Click to see full answer. To be a metal, one or more loosely held electrons has to become “an electron ocean” within which the cationic ion is immersed. An element that becomes an ion must have free room for.
Sulfur has characteristics of nonmetals. It is abundant, multivalent, and nonmetallic. It is a poor conductor of heat and electricity, because electrons are not free to move.
Sulphur has characteristics of nonmetals. Sufur is a nonmetal element. It is a poor conductor of heat and electricity because the electrons are not.
Most nonmetals don't conduct electricity, are brittle when solid, lack the luster of metals when solid, and are poor conductors of heat. Many, though not all, metals are also malleable, which means they can be easily shaped and. An element that becomes an ion must have free room for its electrons to move and to create an electric charge.
Even phosphorus, which is to the left of sulfur on the periodic table, and which holds electrons with less tenacity, is not significantly metallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula s8. It does not have luster, it cannot conduct electricity, and it is brittle.
An element that becomes an ion must have free room for. Zn, pb, cu, ag b. To be categorized as a metal, a substance needs to be a good conductor of both heat and electricity.
Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is a non metal study guides chemistry 20 cards to name a monatomic anion change the suffix of the element's name to the electron geometry of a water molecule is even though the molecular. Sulfur (s), also spelled sulphur, nonmetallic chemical element belonging to the oxygen group (group 16 [via] of the periodic table), one of the most reactive of the elements.
At room temperature it is a yellow crystalline solid. The group is called the oxygen group or the chalcogens. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula s 8.
Even though it is insoluble in water, it is one of the most versatile elements at forming compounds.
Sulfur (s) is an element that can never be overlooked. Which statement is true for one molecule of sulfur trioxide?
What Is The Molecular Mass Of Sulphur? - Quora
The name oxygen comes from the greek stems oxys , acid, and gennan, to form or generate. thus, oxygen literally means acid former.

One molecule sulfur. So yeah, we now did an option. Oxygen is the most abundant element on this planet. Liquid c7h8o is burned with oxygen gas to produce gaseous carbon dioxide and water vapor balanced…
So which one has more balls? Or we have one more love self try oxide which is which will be equal to 80 g. Basically, access which of the following substances have more wars of sulfur.
Multiply the number of moles by 6.022×10^23 atoms/mole. Yeah, yeah, to move verses. Our heaviest item how heavy is compound.
Number of atoms in one molecule of sulphur: 2 see answers advertisement advertisement missaann missaann But molecular weight is not an integer (except for carbon 12).
Reset 1 see answer kaitlyncolbird is waiting for your help. So in this case, there's going to be this one be here. There… get the answers you need, now!
Total number of atom in one molecule of sulfur is____. What is one atom of sulfur reacts with one molecule of oxygen to form one molecule of sulfur dioxide? So the number atoms in each sulfur is 8.
And option we have one more oxygen. It means one more of oxygen is equal to 32 g. Sulfur dioxide, so2, reacts with oxygen, o2, to form sulfur trioxide, so3 sulfur trioxide then… a:
In colour, it is bright yellow, and it has an extremely bad odour (like rotten eggs). Sulfur (s), also spelled sulphur, nonmetallic chemical element belonging to the oxygen group (group 16 [via] of the periodic table), one of the most reactive of the elements. Mahuyamukherjee555 mahuyamukherjee555 3 weeks ago science secondary school +5 pts.
Pure sulfur is a tasteless, odourless, brittle solid that is pale yellow in colour, a poor conductor of electricity, and insoluble in water. The molecular weight of oxygen (o2) is : One mole of so2 contains 1 mole of s atoms and 2 moles of o atoms, for a total of 3 moles of atoms.
There is one atom of sulfur and one atom of oxygen. 3 moles × (6.022×10^23 atoms/mole) = 1.807×10^24 atoms to four significant figures there are ~1.807×10^24 atoms in one mole of so2. Which statement is true for one molecule of sulfur trioxide?
Some basic concepts of chemistry book:aakash institute. Sulfur dioxide = so2 sulfur trioxide = so3 sulfuric acid = h2so4 silver sulfide = ag2s q: See answer (1) best answer.
I mean molecular mass of the given molecule is 80 g. Answered one molecule of sulphuric acid is made of 2 We have one mole of oxygen.
There is one atom of sulfur and three atoms of oxygen. There is one atom of sulfur and one atom of oxygen. Calculate mass of one molecule of sulphur dioxide (so_(2)) in gram.
In this case, which one's more? 1 mole of anything is 6.022×10^23 things, including atoms. One sulfuric (h2so4) molecule has 2 hydrogen atoms, 1 sulfur atom, and 4 oxygen atoms.
Four for most of us. Number of atoms in one molecule of sulphur a. You can also say one mole of sulfuric acid has two mols of hydrogen atoms, 1 mol of sulfur atoms, and 4 moles of oxygen atoms.
The earth's crust is 46.6% oxygen by weight, the oceans are 86% oxygen by weight, and the atmosphere is 21% oxygen by volume. We will update the answer very soon. Click here👆to get an answer to your question ️ number of atoms in one molecule of sulphur:
Our expert is working on this class x maths answer. It is located in group six or sixteen and period three of the periodic table. Hence option a is correct.
It's basically just going to be the same in both above, so no. Add your answer and earn points. There is one atom of sulfur and three atoms of oxygen.
In the periodic table, sulfur is found in group 16.
After this step, the oh is now turned into a good. Chlorides, bromides, and tosylate / mesylate groups are excellent leaving groups in nucleophilic substitution reactions, due to resonance delocalization of the developing negative charge.

Tosylates And Mesylates – Master Organic Chemistry
Article 258 tfeu enforcement process;
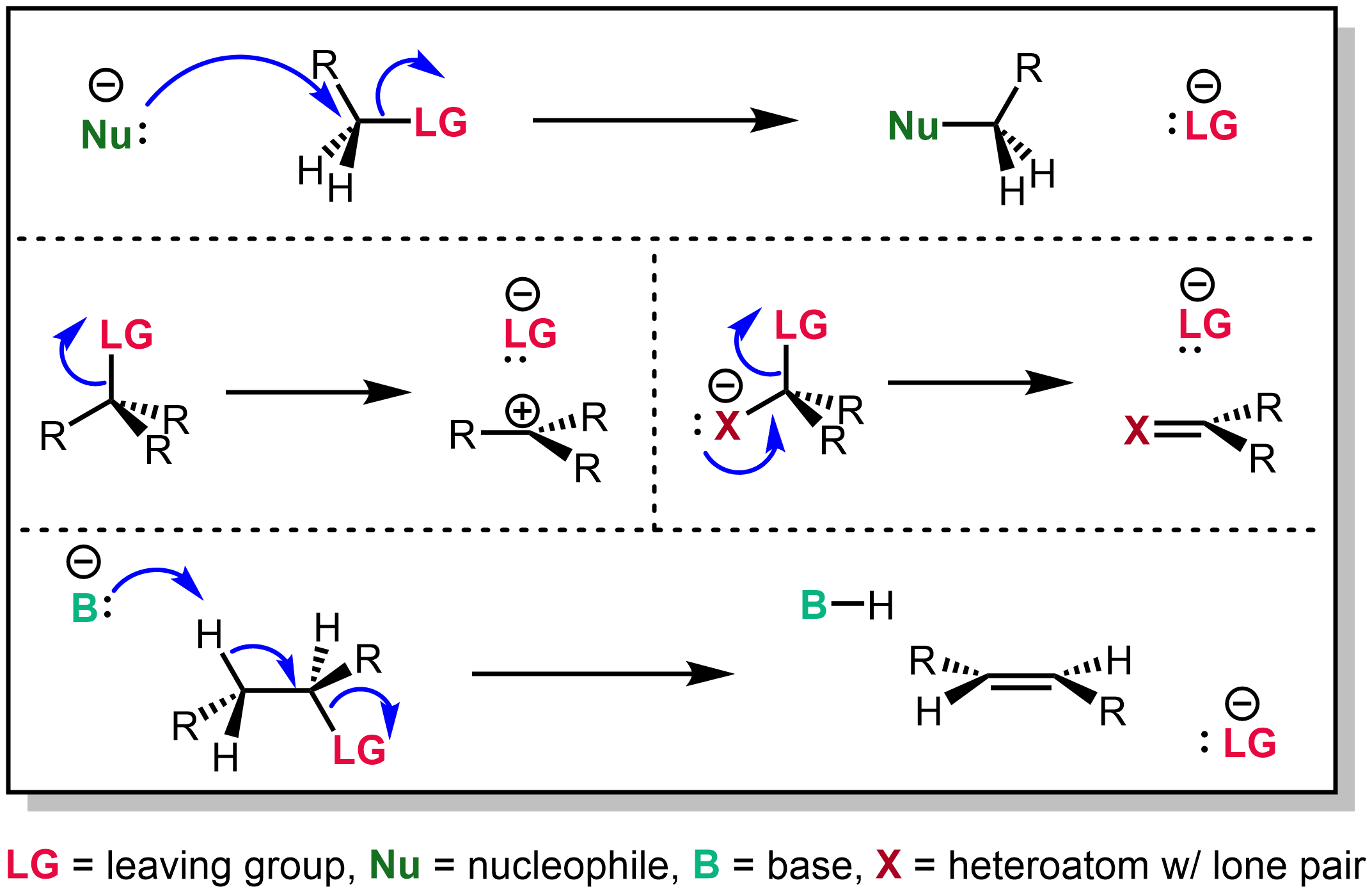
Why are tosylates good leaving groups. Tosylates have a much better leaving group than the original alcohol: Hence, another advantage of using tosylate as protecting group for alcohols is that the alcohol can be reobtained. As we learned previously, resonance stabilized structures are weak bases.
Alkyl sulphates and sulphonates make excellent leaving groups because of this. Tobacco road marathon 2021 results; Resonance increases the ability of the leaving group to leave:
Explain why tosylates make good leaving groups. Tosylate anion also forms stabilized resonance structure on leaving and hence is an excellent leaving group. Triflate, tosylate, and mesylate ions are excellent leaving groups, because the sulfonate ions can stabilize the negative charge via resonance.
Explain why tosylates make good leaving groups. These reactions are favourable for alkyl halides, as halogens are (usually) good leaving groups (halide ions are weak bases). Chlorides, bromides, and tosylate / mesylate groups are excellent leaving groups in nucleophilic substitution reactions, due to resonance delocalization of the developing negative.
$\begingroup$ although i appreciate that brosylates are more prone to solvolytic reactions than tosylates, your link, which reads (remember, brosylate is p. Duncan hines dark chocolate fudge cake mix cookies; Fliers' military branch crossword clue
Alcohols can also react through elimination and. The second arrow always shows a pair of electrons going toward the. The conversion to a sulfonate prevents the alcohol from acting as an acid or nucleophile, or from.
Injecting apple juice in pork The pyridine is added as a base to deprotonate the intermediate and speed up the process of forming the toluenesulfonate ester (tosylate). What is the orientation of the stereogenic center after the reaction depicted in the figure?
Tosylate is a good leaving group. Good leaving groups are weak bases. Csgo dust 2 collection 2021 odds;
Consider a general nucleophilic substitution reaction. What is the orientation of the stereogenic center after the reaction depicted in the figure? Tosylates and mesylates are widely used in the protection of alcohols.
Tosylate groups not only act as.
Is li2n ionic or covalent? When the difference in electronegativity is more than 1.7, the bond is said to be ionic.

Is Lif Ionic Or Covalent/Molecular? - Youtube
Between 100% covalent bond and 100% ionic.

Is li ionic or covalent. Downvote + physical chemistry + chemistry. Therefore, the statement, licl is covalent while nacl is ionic is true. Li will donate its 2s electron to cl and both become ions (charged atoms).
Licl is an ionic compound but it also has small covalent characteristics due to the small size of lithium. Lithium chloride is an ionic compound but it also has some covalent character due to the very small size of lithium metal. The phosphorus is bonded to three hydrogen atoms and has a lone pair of electrons.
541k subscribers there are two ways to tell if licl (lithium chloride) is ionic or covalent. But you should also know that there is no clear strick line to determine between ionic and covalent bond. If the electronegativity difference between the atoms forming a bond lies between 0.5 to 2.0, it is considered a polar covalent bond.
Lithium nitride is the only known thermodynamically stable alkali metal nitride and is one of the most ionic of all known nitrides. Home > community > is li2cro4 ionic or covalent? It has the formula ph3.
Lithium is a metal, chloride is a nonmetal. What type of bond is li and o? Does licl have a covalent bond?
Chemical bond a chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. The polarising power of lithium is high so it has covalent characteristics. In multiple covalent bonds, two or three pairs of electrons are shared by two atoms.
Amongst li and c, c is more electronegative. So, licl is an example of an ionic bond. 0 followers · 0 following joined december 2019;
As you look at licl, looks to be ionic because of the electrostatic forces of attraction between positive and negative ions. Some covalently bonded atoms also have lone pairs, that is, pairs of valence electrons that are not involved in bonding. Ionic, although with small cations and large anion there will be some polarisation of the anion and some covalent character to the bonding.
Lif ( lithium fluoride ) is ionic what is chemical bond, ionic bond, covalent bond?
This infact is a brutal and painful example of osmosis , not beautiful at all. Answer verified 116.1k + views hint:

Real World Examples Of Osmosis. Example 1 Sore Throat? Gargle Salt Water. Why? Swelling Throat Tissues, Contain H 2 O Salt Water Has Lower Concentration. - Ppt Download
Unlike diffusion, which may occur in any media (gas, liquid, or solid), osmosis occurs exclusively in liquids and (rarely) gases.
What is a real life example of osmosis. A common osmosis example seen in real life is the preservation of food. List of 25 important biology topics for competitive exams list of neet questions to crack neet 2021 Osmosis refers to the movement of one, less concentrated solvent through a semipermeable membrane to another, more concentrated solvent.
The potato acts as a membrane, and after a while it will be seen that the solution that had sugar now has more liquid. To better explain this phenomenon, we have listed a few very good examples of osmosis that we encounter in everyday life. Feeling thirsty after having salty food.
Cells have membranes which do not allow salt in and out. Plants absorb water from the soil. A common experiment of osmosis is to split a potato, placing at one end a bit of sugar with water, and on the other a dish with water.
During the day, plants exhale oxygen which we take and we exhale co2 which are taken by plants. What is a real life example of osmosis? What are real life examples of osmosis?
Osmotic pressure answer what are some examples of osmosis in real life? It is observed in resin (kismis) or kabuli gram ( chana) when we put in water through osmosis they get expand. Diffusion occurs when molecules tend to spread themselves uniformly in space, as in osmosis.
Sprinkling salt of earthworm causes its cells to dehydrate just to main equilibrium between the high concentration of salt outside the cells. If you are there in a bath tub or in water for long your finger gets pruned. A real life example is the chloroplasts found in plant cells.
Reason behind it is osmosis. Fish absorb water through their skin and gills. Here are 6 reverse osmosis examples in real life.
Two containers a and b are filled with equal volume of water and are separated by a membrane that allows water to pass freely, but prohibits passage of solute molecules. The amounts of freshwater they produced were too little to be of use. How do animal cells involve osmosis?
Red blood cells placed into freshwater. Solution a has 3 molecules of albumin protein (molecular weight 66,000) and solution b has 15 molecules of glucose (molecular weight 180). Dialysis of kidney in the excretory system.
Terms in this set (14) diffusion. Movement of water and minerals from root nodules to various parts of plants. Swelling of resins and other seeds when they are soaked in water.
What is a real life example of osmosis? Another example of osmosis is that our fingers get pruned after being inside water for a long time. One of the best examples of osmosis is that dry grapes get puffed when immersed in water.
Desalination we have come a long way since the days scientists first attempted to desalinate ocean water in the 1950s. For animal cells one would be when ur skin gets wrinkly after being in water for long periods. However, using cellular acetate as a membrane, the process is now faster and efficient.
What are some real life examples of osmosis? The adh hormone that allows the reabsorption of water by the collecting tubule, in the kidneys. Plants take water and mineral from roots with the help of osmosis.
The phenomenon behind this is osmosis. Food is preserved in salt or sugar solution (hypertonic) to allow water to be pulled out of the cells of the foods. In a hypertonic solution, when we soak almonds in water, they swell out as the water in the container flows from there to the almonds.
The dot structure for the chlorite ion has the cl atom in the center with a double bonded o atom with two double. The oxygen atom with a single bond has three lone pairs, and the oxygen atom with a double bond has two lone pairs.

Chemistry Net: Simple Method For Writing Lewis Structures: Chlorine Dioxide Clo2
1 cl + 2 o + 1 e = 1 ×7 +2 ×6 + 1 = 20.

Lewis structure clo2. These four electrons form a double covalent bond between carbon and oxygen atoms. The chlorine atom has 2 lone pairs, one oxygen atom has 2 lone pairs and the other oxygen atom has 3 lone pairs. When the co2 lewis structure is examined, it is seen that two pairs of electrons are shared between the carbon and oxygen atoms (a total of four electrons).
The lewis dot structure for clo2 has one double bond and one single bond. Lewis structure of clo2 (or chlorine dioxide) contains two double bonds between the chlorine (cl) atom and oxygen (o) atom. This means that the number of valence electrons is 14.
No lone pairs on carbon atom and each oxygen atom has two lone pairs on their valence shells. For clo2, all elements will have 8 electrons. So, you can draw the co2 lewis structure in this way.
Hence, the trial structure has the correct number of electrons. The valence electrons you have available are: There are total of 20 valence.
There are 2 lone pairs on double bonded oxygen atom (o) and 3 lone pairs on single bonded oxygen atom (o). The left oxygen atom has two lone pairs, the right oxygen atom has three lone pairs, and the chlorine atom also has two lone pairs. There is 1 single bond and 1 double bond between the chlorine atom (cl) and each oxygen atom (o).
For the lewis structure for clo2 you should take formal charges into account to find the best lewis structure for the molecule. Each oxygen atom has two lone pairs, and the chlorine atom has one lone pair and one unpaired electron. Lewis structure of clo2 clo 2 (chlorine dioxide) has one chlorine atom and two oxygen atoms.
The clo2 lewis structure has 19 valence electrons meaning that there will be an odd number of valence electrons in the structure. The trial structure is you have 20 valence electrons in your trial structure. Carbon dioxide (co 2) lewis structure has two oxygen atoms and one carbon atom.
In the lewis structure of the compounds, the valence electrons are denoted by dots. Leave a comment / chemistry / by admin. Two oxygen atom participates in the formation of clo2 therefore, the total of number of valence electron in clo2 is (7+ (2*6)) = 21.
There are two double bonds around carbon atom in the co 2. Draw lewis dot structure of a chlorite ion clo2? What is the lewis structure for clo2?
A lewis structure is a very simplified representation of the valence shell electrons in a molecule. National center for biotechnology information. Remember that the negative sign counts as one valence electron.
Shape of co 2 is linear. Clo 2 has an odd number of valence electrons (19). In the lewis structure of clo 2, there are two double bonds around the chlorine atom, with two oxygen atoms attached to it.
That means that the chlorine (cl) atom will have an unpaired electron and therefore won't have an octet (it will have seven in clo 2. The chlorine atom has 1 lone pair and 1 unpaired electron, while both the oxygen atoms have 2 lone pairs. The chlorine atom (cl) is at the center and it is surrounded by 2 oxygen atoms (o).
Electrons are shown as dots or for bonding electrons as a line between the two atoms. The number of valence electron in chlorine is 7 and in oxygen is 6. For the lewis structure for clo 2 place chlorine (cl) in the center of the structure since it is the least electronegative.
It is used to show how the electrons are arranged around individual atoms in a molecule. The chlorine atom (cl) is at the center and it is surrounded by 2 oxygen atoms (o). Steps of drawing the lewis structure of co 2 are explained in detail in this tutorial.
This website uses cookies to improve your experience, analyze traffic and display ads. The period of y=tanx is π.

Tangent Function - Formula, Properties, Faqs | Tan Graph | Tan X
Look at the graph closely.

Tan x period. The general formula of trigonometric graphs are a trigo function bx + c so for example, in this case a=1 (amplitude), trigo function is tangent, coefficient of x is 2 (b=2) and there is no constant c, so c=0. You can put this solution on your website! Given below is a graph of the tan function.
But notice it is not mirror symmetric around any other point. Tan pi/4 and tan (pi/4 + pi) have the same value (1). Y = tan x period:
Tan α = a / b example a = 3 b = 4 tan α = a / b = 3 / 4 = 0.75 graph of tangent tbd tangent rules inverse tangent function Y = tan x firstly, let's observe: 4) the graph of tan(x) is symmetric with.
Share answered may 22, 2013 at 19:43 angela pretorius 10k 1 24 42 add a comment /b where is the coefficient of x /b where is the coefficient of x In the above graph, the x axis denotes the angle,.
Does tan have a period of 2pi? 2 x / π ∉ z }, that is to say, all reals which are not integer multiples of π / 2. Trigonometry graphing trigonometric functions circular functions of real numbers 1 answer massimiliano jan 29, 2015 the period of y =.
The period of tan2x is 2π. So you can't shift the graph through a period so it looks exactly the same. What is the period of |tan2x?
Referencing the figure above, we can see that each period of tangent is bounded by vertical asymptotes, and each vertical asymptote is separated by an interval of π, so the period of the tangent function is π. Y = tan 2x period: A = 1 a = 1 b = 1 b = 1 c = 0 c = 0 d = 0 d = 0 since the graph of the function tan t a n does not have a maximum or minimum value, there can be no value for the amplitude.
Compared to y=tan(x), shown in purple below, which has a period of π, y=tan(2x) (red) has a period of. Tan calculator tangent definition in a right triangle abc the tangent of α, tan (α) is defined as the ratio betwween the side opposite to angle α and the side adjacent to the angle α: The period of tan x is pi.
On this domain, we can state. Find amplitude, period, and phase shift y=3tan (x) i am unable to solve this problem. I got the feeling that it was periodic, but actually tan|x| isn't.
3) the domain of tan(x) is the set of all real numbers except x = π 2 + nπ , n being any integer. How do you find the period of tan x? 0 0 similar questions 1+sec2xtan2x is equivalent to hard view solution > find the.
A → b is periodic with period p if, for all x ∈ a, f ( x + p) = f ( x) and p is the least such value for which this is true. This means that, the graph repeats itself every π radians. It should have a mirror symmetry around x=0.
Period for tangent graph is different from sine and cosine. This is because tan is the y value divided by the x value at any given point on the unit circle. /2 y = tan (1/2)x period:
Solution verified by toppr correct option is c) let f(x)=tan(x+4x+9x.+nx) =tan( 6n(n+1)(2n+1)x). Solution verified by toppr the period of tanx is π. ( using ∑ r=0n r 2= 6n(n+1)(2n+1) period oftanx=π ∴ periodof f(x)=.
Tangent function formula now, we have two main formulas for the tangent function. The tangent function is periodic on the set of real numbers r with period π. 1) tan x has a period equal to π.
The period for tan(x) is π radians or 180⁰. Qual é o período de # y = sin2x #? 2) tan(x) has vertical asymptotes at all values of x = π 2 + nπ , n being any integer.
The “fundamental” branch is in (−π2,π2). You can see it on the graph: The tan function has a period of π radians (or 180⁰).
I saw a lot of functions, resembling tan (x). Y = 3 tan x graph and label for 2 cycles At 0 degrees, x is at one and y is at zero, so tan0o.
Tangent function tan x is a periodic function and has a period of π/1 = π (because b =1 in tan x). A = { x ∈ r: What is the period of y = tan x?
However, if f(x) = tan(x) for a < x < b, where a > − π 2 and b < π 2, the periodic etension of f(x) has a fourier transform.
If you cross a red and a red flower and you come up with white what. Asiatic lily ‘black out’ (with.

26 Types Of Pink Flowers: Tips + Pictures - Proflowers Blog
Abelia × grandiflora abutilon megapotamicum acalypha hispida acca sellowiana.

Red flower white flower end up with pink flower. Red + white = pink, no matter what type of mixing or crossing occurs. Choose from a wide array of neon colors to accent the pink: Shop the best in artificial flowers and fake plants.
This type of hibiscus shrub produces large graceful flowers that are pink, white, red, or yellow. Each leaf is rounded and can grow to breadths of from ¼ to 1 inch. Rose of sharon is a hardy hibiscus that belongs to the rosaceae family of flowering.
Petunias are an autumn or fall flower that produces mainly pink, white, and violet blossoms. They will also attract bees,. Red flowers represent desire, strength, and.
Planting chrysanthemums can give your garden splashes of white, red, purple, or pink in the fall. Wether you are looking to style fake flowers, pampas grass. They are native to the higher altitudes of the.
Afloral offers premium artificial plants, silk flowers, dried flowers and vases. The following photos will allow you to identify red and pink flowering plants. These showy flowers come in varying shapes and sizes.
A flower, sometimes known as a bloom or blossom, is the reproductive structure found in flowering plants (plants of the division angiospermae).the biological function of a flower is to. The fragrant flowers, which range from red to pink to white are 2 to 5 inches wide and bloom in the winter. Click on image to view plant details.
Plant in full sun or partial shade. This is a flower that is loved by. Red clover is a very common garden weed that you’ll often find growing on your lawn.
It’s immediately recognizable by its dark pink flowers. Each flower head consists of 40. Stunning beautiful red flowers that are perfect as wedding flowers or other celebrations.
Red sunset large plants true red backyard landscaping early spring ever red® was the first variety of loropetalum on the market to produce red flowers, instead of pink or white. The cornflower (bachelor’s button) is a colorful flower that makes a beautiful addition to your garden and can grow in blue, white, red, pink, and purple. Having petunias in your garden will give it plenty of color in the fall when some.
(a) 16(b) 10(c) 9(d) 8 Therefore, the valency of sulfur is 2, 4, 6.

How To Draw The Lewis Dot Structure For S 2- (Sulfide Ion) - Youtube
The following solution is suggested to handle the subject “1.determine the bond order in a molecule or ion with 10 valence electrons.
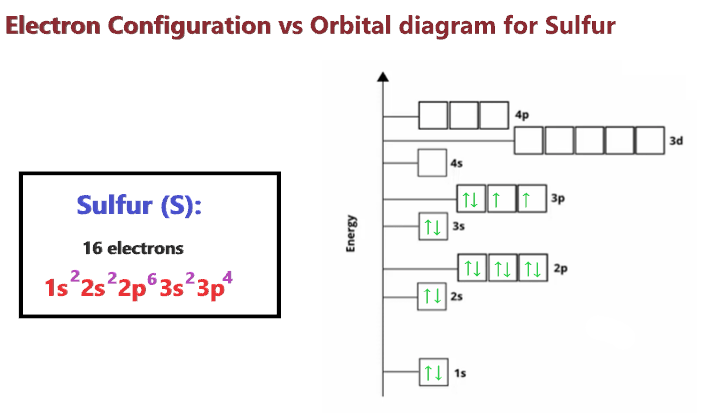
Valence electrons of s2- ion. Solution option (d) the sulfur atom has 6 valence electrons, and when it gains 2 more electrons to form s2−, then it has 8 valence electrons. How many valence electrons does sn 2+ have?. It has 6 valence electrons in its outer energy level, however,.
From the above information, we can say that sulfur exhibits variable valency. A sulfide ion has a full complement of 8 valence electrons. Therefore, the number of valence electrons in sodium ion is 8.
4 s 2 3 d 2c. How many valence electrons does a sulfide ion have? A 0.2 n a b 3.2 n a c 3.6 n a d 2.8 n a medium solution verified by toppr correct option is d) valence e − in o 22−=2×6+2[e.c.=2,6] ∴ total no.
So the valency of sulfur is 6. In nitrogen (n) atom having atomic no.7,so electronic configuration = 2,5. Seamlessly assign resources as digital activities.
The 8 valence electrons in the sulfide ion encounter a large amount of repulsion between electrons than the 6 valence electrons in the neutral sulfur atom. How many number of valence electrons are there in a sulphide ion? Neutral sulfur has 6 valence.
Concept notes & videos 313. Question total number of valence electrons present in 6.4 g peroxides ion (o 22−) is ______________. Question “1.determine the bond order in a molecule or ion with 10 valence electrons.
3 p6 4 s2 login study materials ncert solutions ncert solutions for class 12 ncert solutions for class 12 physics ncert solutions for class 12 chemistry ncert solutions for class 12 biology ncert solutions for class 12 maths Cbse cbse (english medium) class 9. The electron configuration of the sulfide anion will thus be s2−:a1s22s22p63s23p6
Now, the sulfide anion, s2−, is formed when two electrons are added to a neutral sulfur atom. In na + ion, there are only 10 electrons. 4 s0 3 d 4b.
Of valence e −= 326.4×n a×14=2.8 n a option (d) is correct. Compound formation of sulfur sulfur participates in the formation of bonds through its valence electrons. Learn how in 5 minutes with a tutorial resource.
Read free for 30 days. 4 s2 3 d 0d. The electronic configuration of sulfur ion is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6.
Sulfide ion is formed by the two electrons. Browse valence electrons and ions resources on teachers pay teachers, a marketplace trusted by millions of teachers for original educational resources. Let’s keep an eye on the content below!
This electron configuration shows that the last shell of the sulfur atom has six unpaired electrons. The valence shell electronic configuration of cr 2+ ion is:a. The electronic configuration is 2, 8.
The electronic configuration is 2 , 8. But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.
60 n at 90 degrees 2. A ball (mass m = 250 g) on the end of an ideal string is moving in a circular motion as a conical pendulum.
Express each of the following in terms of another angle between 0 degrees and 180 degrees a.

Tan 150 degrees. For all trignometric value(sin cos tan cosec sec cot) we have a table of ratios for each sin cos. Tan 120° in terms of trigonometric functions using trigonometry formulas, we can represent the tan 120 degrees as: It is called tangent since it can be represented as a line segment tangent to a circle.
The value of tan 945° is. Sign up to explore more. Tan 150 degrees 22,180 results, page 2.
And therefore we can say that the sine of one degree is the same thing as sign of five pi over six, because in the. 40 n at 0 degrees 3. If the angle is not any of those like 0 30 45 60 or 90 then also we can find the ratio but not directly but with some formula, such as sin 75 = s.
How do you find the value of tan 150? In this video, we learn to find the value of tan150. The url of the video.
Tan(150) = tan(180 −30) or tan(150) = tan(180 +( − 30)) tan(150) = tan(180) +tan( − 30) 1 − tan(180.tan( − 30)) tan(150) = 0 + sin(−30) cos(−30) 1 − 0 tan(150) = 1 2 − √3 2 How do you find the value of tan 150? Sign up to explore more.
Cos 120 degrees please explain to me how to answer this question you don't have to answer it. The value of tan 180 degrees is equal to 0. 50 n at 60 degrees what are the magnitude and direction of a sixth force that would produce equilibrium?
We would love to personalise your learning journey. Tan (150) tan ( 150) apply the reference angle by finding the angle with equivalent trig values in the first quadrant. That’s all you get for now.
In this video, we learn to find the value of tan150. Make the expression negative because tangent is negative in the second quadrant. Click here👆to get an answer to your question ️ how do you find the value of tan 150?
Since our angle is greater than 90 and less than or equal to 180 degrees, it is located in quadrant ii in the second quadrant, the values for sin are positive only. 80 n at 270 degrees 4. Checkout jee mains 2022 question paper analysis :
Exact value of tangent of 150 degrees as a fraction calculator chart examples find the exact value of tangent of 150 degrees as a fraction. Tan (150°) tan ( 150 °) apply the reference angle by finding the angle with equivalent trig values in the first quadrant. In the graph above, tan (α) =.
Tan (1 5 0) = tan (1 8 0 + (− 3 0)) tan. Trigonometry right triangles trigonometric functions of any angle 1 answer टासी श. Learn how to find the value of tan 180 degrees and how to derive the formula for tan 180 minus theta, here at byju’s today!
Five forces act on an object: 40 n at 180 degrees 5. · mimi oct 17, 2015 1 −√3 explanation:
Make the expression negative because tangent is negative in the second quadrant. This website uses cookies to improve your experience, analyze traffic and display ads. Tan (1 5 0) = tan (1 8 0 − 3 0) or.
That’s all you get for now. How to find the value of tan 150°? We would love to personalise your learning journey.
Video answer:so the question asked to do the change of 150° and to show all steps so we know that the tangent of any angle is going to be signed over cosign. And we can find the ratio easily.
Most chlorite minerals are green in color, have a foliated appearance, perfect cleavage, and an oily to soapy feel. Sodium chlorite is a strong oxidant and can therefore be expected to cause clinical symptoms similar to the well known sodium chlorate:

Clo2 Lewis Structure (Chlorine Dioxide) - Youtube
Show transcribed image text expert answer.

Chlorite lewis structure. How many charges in atoms of chlorate ion lewis structure? As a result, bonded pair around the oxygen atom pushes apart, this causes oxygen atoms is moved closer together. The valence electrons you have available are:
In the lewis structure of the compounds, the valence electrons are denoted by dots. This problem has been solved! Two oxygen atom participates in the formation of clo2 therefore, the total of number of valence electron in clo2 is (7+ (2*6)) = 21.
In this ion, the chlorine atom does follow the octet rule, unlike clo 3−, or clo 4−. The number of valence electron in chlorine is 7 and in oxygen is 6. See the answer see the answer see the answer done loading.
Oxygen atom is the center atom and both chlorine. Assign a formal charge to each atom in the student's lewis structures. It contains two chlorine atoms and one oxygen atom and we will learn how to draw the lewis structure of cl 2 o step by step in this tutorial.
Draw the lewis structure for the chlorite ion (cio2) with minimized formal charges. Chlorite minerals are found in rocks altered during. The lewis structure is a pictorial representation of valence electrons taking part in the formation of bonds to produce a new molecule with new properties altogether.
Dichlorine monoxide is an one oxide of chlorine. 70 more lewis dot structures. Click to edit molecule a) 1 b) 2 c) 3 d) 4 e) 5.
The trial structure is you have 20 valence electrons in your trial structure. The oxygen atom with a single bond has three lone pairs, and the oxygen atom with a double bond has two lone pairs. All other atoms do not have charges.
To use the lewis structure calculator follow these steps: Enter the formula of the molecule in the field provided for it. For chlorine, atomic number = 17
∴ there are 4 atoms or ions present in each unit cell of a face. 1 cl + 2 o + 1 e = 1 ×7 +2 ×6 + 1 = 20. Cl 2 o (dichlorine monoxide) lewis structure and steps of drawing.
They are found in igneous, metamorphic and sedimentary rocks. [17] methemoglobemia had been demonstrated in rats and cats, [18] and recent studies by the emea have. Chlorite is the name of a group of common sheet silicate minerals that form during the early stages of metamorphism.
Therefore, shape of ion is trigonal pyramidal. Hence, the trial structure has the correct number of electrons. Science chemistry q&a library draw the lewis structure for the chlorite ion (cio2) with minimized formal charges.
To begin drawing the lewis structure of chlorine dioxide, first, it is essential to draw one for the participating elements. How many total likely resonance structures exist for cio2 ? (assign lone pairs, radical electrons, and atomic charges where appropriate.) this problem has been solved!
Remember that the negative sign counts as one valence electron. Put the least electronegative atom in the center. See the answer show transcribed image text.
The corner site of the nacl crystal structure is shared by the 8 unit cells (1 corner equal to 1/8 of ion or atom) and the 6 face site is shared by 1/2 atoms or ions.
The capacitance is directly proportional to the surface area of the objects and the dielectric constant of the material between them, and inversely proportional to the distance between them. D = displacement π = pi (3.14) b = bore s = stroke c = number of cylinders it might appear to be complex, but it is actually pretty easy to understand how it was derived, if you know what bore and stroke mean.

How To Calculate Work: 11 Steps (With Pictures) - Wikihow
If you have dozens of connectors and/or dozens of steps or subcases, this would be a easy way to get all the results in a table.

How to measure work displacement. How to measure displacement by correlation. Learn more about daq, data acquisition Capacitive displacement sensors operate by measuring changes in electrical capacitance.
Learn more about doit4me, sendit2me This video shows how to use the water displacement method to. A linear potentiometer is simply a variable resistance.
Measuring cylinders help in finding volume of liquids, but what of bodies with irregular shapes? This person is not on researchgate, or hasn't claimed this research yet. The capacitor, which is present in above circuit has two parallel plates.
The following is a brief explanation of both these quantities. In physics terms, you often see displacement referred to as the variable s. How does capacitive displacement sensor work?
However, being familiar with the nastran file is helpful. Measurement of displacement using capacitive transducer the circuit diagram of capacitive transducer, which is used to measure displacement is shown in below figure. Accurately measure displacement in harsh conditions an inductive encoder measures a displacement relative to a target.
The displacement is translated into a movement. In physics, you find displacement by calculating the distance between an object’s initial position and its final position. Read 1 answer by scientists to the question asked by rakan alalwan on jan 11, 2021
Among which, one plate is fixed and the other plate is a movable one. The following is the formula used for measuring an engine’s displacement. Whether either of those is easier depends on the situation.
A displacement cylinder operates by oil displacing the cylinder rod. How to measure the displacement with. With a displacement hydraulic cylinder, the rod pushes on the outward stroke only and the cylinder relies on the load or some other force to return the cylinder to the closed position.
The official displacement formula is as follows: The simplest and cheapest way to measure displacement electrically in geomechanics applications is using a linear potentiometer. The resistor typically consists of a ceramic material that has an.
Join / login >> class 11 >> maths >> limits and derivatives >>. Here is a list of the derivatives that you need to know:

Calculus - The Derivative Of $ \Tan X$ Is $ \Sec^2 X$. Why? - Mathematics Stack Exchange
Provided that you simply want the derivative of r with respect to the variable θ, rather than something like the derivative of x(θ) or y(θ) with respect to θ, you simply.

Differentiate x=tan theta. It can be proved by the definition of. Note tan2x = (tanx)2 differentiate using the chain rule given y = f (g(x)) then dy dx = f '(g(x)) × g'(x) ← chain rule y = (tanx)2 ⇒ dy dx = 2tanx × d dx (tanx) ⇒ dy dx =. Derivative of the tangent squared function.
The formula for differentiation of tan x is, d/dx (tan x) = sec2x (or) (tan x)' = sec2x now we will prove this in different methods in the upcoming sections. Click here👆to get an answer to your question ️ if x = asectheta, y = btantheta then dydx = solve study textbooks guides. As z = 0, with ∣z∣ = 1 z2 + 1z2 − 1 = z + z1z − z1 now z1 = cosθ +isinθ1 = cosθ − isinθ.
See the answer see the answer see the answer done loading View solution steps evaluate tan(θ) graph quiz trigonometry tan(θ) similar problems from web search find tan(θ) where θ is the angle between v and w. Understanding this solution for a trigonometric identity of tan2θ.
Complex number problem itan(θ) proof. If x=a(θ+sinθ) and y=a(1−cosθ), find dy/dx. The derivative of with respect to is.
To differentiate the tangent function, tan(x), follow these rules. Easy solution verified by toppr x=a(θ+sinθ) ⇒ dθdx=a(1+cosθ) (1) y=a(1−cosθ) ⇒ dθdy=a(0−(−sinθ))=asinθ (2) dividing (2) and (1). It is possible to find the derivative of trigonometric functions.
This problem has been solved! You're going to use the product rule, which implies that first you take the derivative of the first time, which is seeking to eggs. We're gonna use the product rule when we get secret.
I have got this same answer earlier on but it doesn't work for some reason. Y = sec θ tan θ. (1/cosx)/ (2sec² (2x) ) = 2cosx/ cos2x so when you sub the pi/6 you dont get 6.
He stayed up of 10 plus tangent data d over deed. To apply the chain rule, set as. Tour start here for a quick overview of the site help center detailed answers to any questions you might have meta discuss the workings and policies of this site
Two terms that are multiplying with each other. In this tutorial we shall discuss the derivative of the tangent squared function and its related examples. The first is to rewrite tan(x) in terms of sines and cosines.
This simply means writing tan(x) as sin(x) / cos(x). So we have why equal? I'll repeat what i said then.
Say we have a function y ( t), that satisfies the ordinary differential equation d y d t = f ( t, y) for t ∈ ( t 0, t max],, where t takes discrete values, t n, with a constant step size h = t n + 1 − t n. Derivative of tan x proof by first.
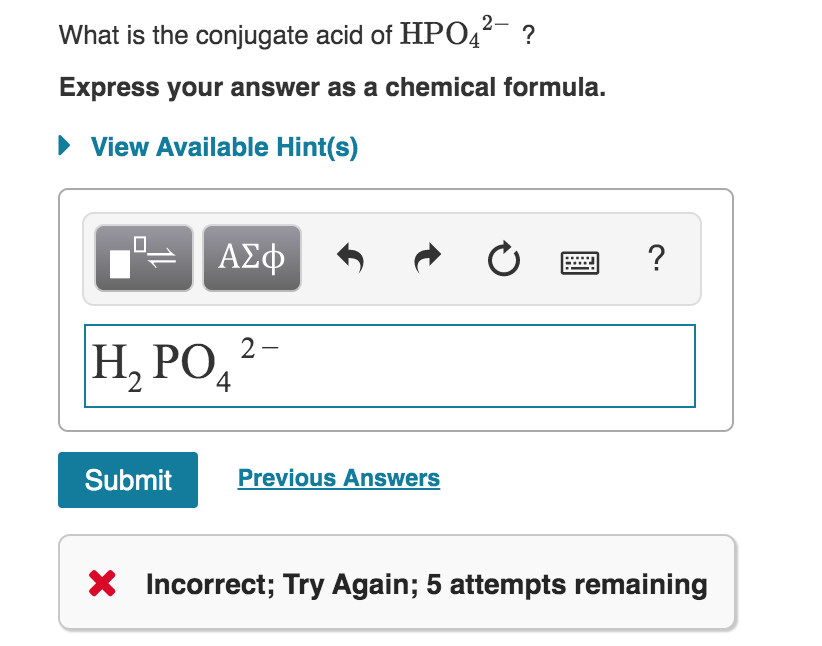
We should know that the conjugate acid of a weak. What is the conjugate base of h2po4?
Solved What Is The Conjugate Acid Of Hpo42- ? Express Your | Chegg.com
If it’s already in water, it will further dissociate.
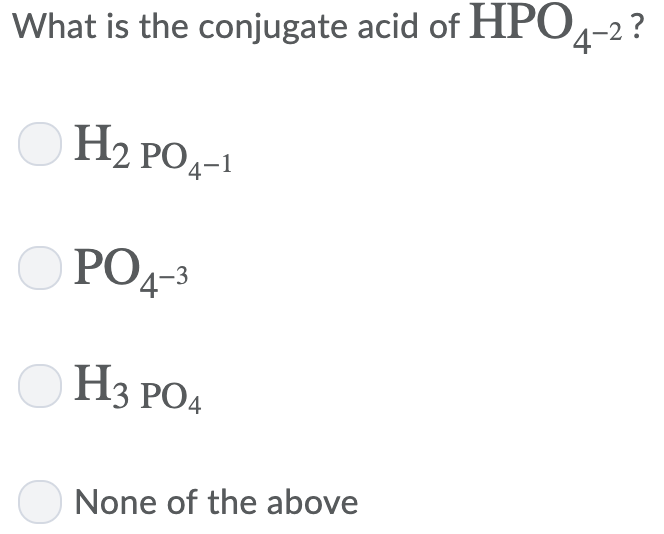
Conjugate acid of hpo4-2. It is the conjugate base of phosphoric acid h3po4 and the conjugate acid of monohydrogen. Or rather phosphoric acid donates a. The conjugate acid of hpo4 2 is h2po4 o true o false.
The conjugate acid of is: Phosphoric acid is the parent acid, i.e. The one hydrogen atom is attached to one of the oxygen atoms with a single covalent bond.
See the answer see the. H 2po 4− d po 43− medium solution verified by toppr correct option is c) the conjugate acid of hpo 42− is h 2po 4− h 2po 4−→h ++hpo 42− the base and its conjugate acid differ. The conjugate acid of hpo4 2 is h2po4 o true o false ;
What best describes a bronsted lowry acid base reaction? H p o 4 − 2 + h + → h 2 p o 4 −. So the correct answer is option c.
Therefore, the base hydrogen phosphate ion reacts with a proton to form a conjugate acid in the following manner: The resulting base is called the conjugate base. What is the conjugated acid of h2po4?
It has to be an acid in molecular form. You cannot put as it is in water. Conjugate acid is h2so4 conjugate base is so42.
Hence, the conjugate acid of h p o 4 −. This problem has been solved! The conjugate base and conjugate acid for hs04 is:
Remove a proton from this, we get, h 2p o− 4 as the conjugate base. This is the best answer based on feedback and ratings. Therefore, the conjugate acid that is formed is h 2 p o 4 −, which is dihydrogen phosphate ion.
The three oxygen atoms are connected to the phosphorus atom with single covalent bonds. By continuing to use this site. Join / login >> class 11 >> chemistry >> equilibrium >> acids, bases and.
Join our discord to connect with other students 24/7, any time, night or day.join here! It is called a common logarithm.

Log10 0.1=-1 In Exponential Form - Brainly.in
I) e and pi are accepted values.
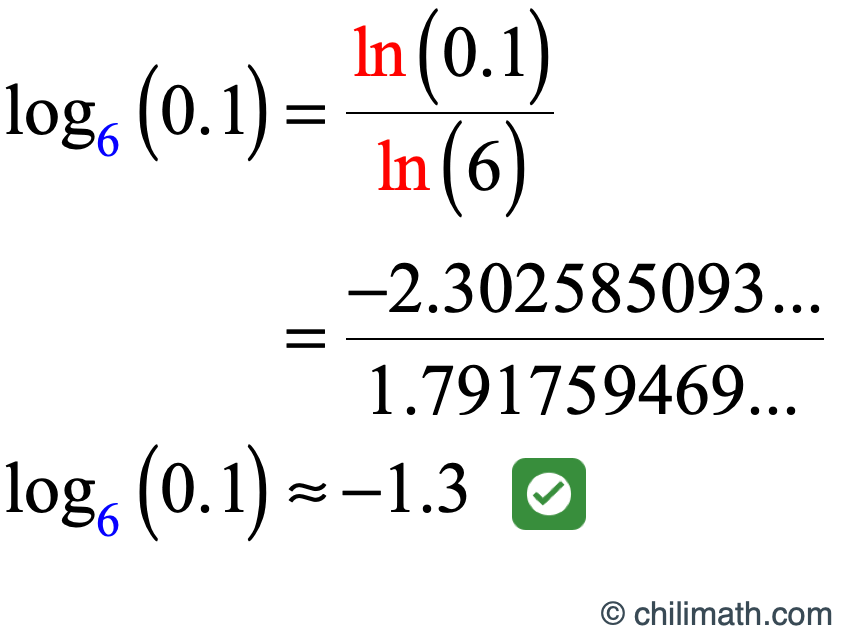
Log of 0.1. Coming after the ninth in numbering or counting order, position, time, etc; Log 2 (8) = log 10 (8) / log 10 (2) see: The change of base rule can be used if and are greater than and not equal to , and is greater than.
💬 👋 we’re always here. Ii) 1.2 x 10 3 should be entered as 1.2e3 and 3 should be entered as 1.2e3 and So here comes log of x log of 10 x and log of 0.1 x.
Log (100) this usually means that the base is really 10. While the natural logarithm is base with the mathematical constant ‘e’, which approximately equals to 2.718281828459 The graph above presents the values for the common, natural and binary logarithm functions for the values from 0.1 to 20 (logarithm of zero is not defined).
Pramod kumar misra 12th in physics, chemistry, and mathematics (science grouping) & schools, central board of secondary education, india (graduated 2016) 4 y Algorithms can be easy to compute in your mind, e.g. I) e and pi are accepted values.
What us importers need to know image type is png. Evaluate log base 0.1 of 100. Sometimes a logarithm is written without a base, like this:
The document is the result of a volunteer community effort. The document includes material on zeek’s unique capabilities, how to install it, how to interpret the default logs that zeek generates, and how to modify zeek to fit your needs. Void usebaseandarg( double argb, double argx ) { // evaluate log(b)[x] == 1 / log(x)[b].
Table of base 10, base 2 and base e (ln) logarithms: The purpose of this document is to assist the zeek community with implementing zeek in their environments. The base b real logarithm of x when x<=0 is undefined when x is negative or equal to zero:
If you follow this advice to add a constant,. Substitute in values for the variables in the change of base formula, using. It looks like what happened was just a.
Log z = ln(r) + i. // example for the math::log( double ) and math::log( double, double ) methods. A different way of seeing this same question is by asking this:
The following example uses log to evaluate certain logarithmic identities for selected values. Engineers love to use it. So log base 10 of 110 to what power is 100 while 10 to the negative.
Rewrite using the change of base formula. Value of log (0.13) home >> area calculate value of log, antilog, natural log (nlog), and exponent of a number. A common logarithm is the logarithm with base 10, also known as the decadic logarithm or decimal logarithm.
Please enter the base (b) and a positive number (n) to calculate log b n: Being the ordinal number of ten: For example, in order to calculate log 2 (8) in calculator, we need to change the base to 10:
So here's my graphing calculator and we go into the y equals menu and we're going to look at the graph of y equals log of x y equals log of 10 x and y equals log of 100.1 x, and i'm just going to use a standard viewing window. It is how many times we need to use 10 in a multiplication, to get our desired number. 10x = 0.1 so the above and log10(0.1)
All right, we're using a graphing utility. Althogh both original values seem to be close to zero, their logarithms are quite different. Log (1000) = log10(1000) = 3.
There's no bass readiness log base 10. On a calculator it is the log button. // evaluate logarithmic identities that are functions of two arguments.
It discusses how to find the oxidation states of elements. , etc) the oxidation numbers are the same as the actual charges on the ions.
Rules For Oxidation Numbers Study Guide | Inspirit
Group 2a elements (alkaline earth metals) always have an.
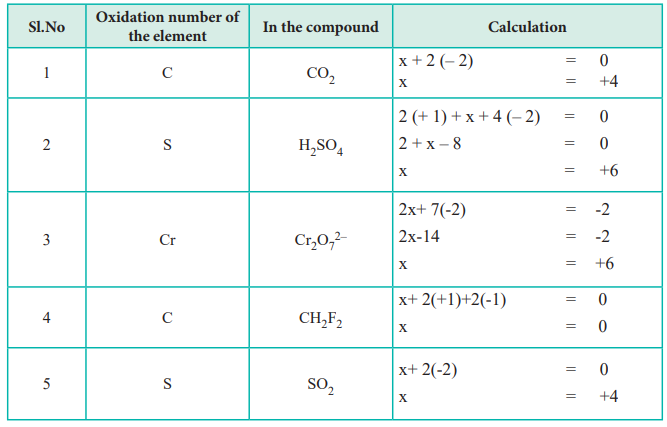
Calculating oxidation number. Charge on an ion is equal to its oxidation number. The sum of the oxidation numbers in a monatomic ion is equal to the overall charge of that ion. The oxidation number of any atom in its elemental form is 0.
It is important to understand the rules to calculate the oxidation number. It is done by keeping the general rule in front; When one atom loses electrons, another atom has to gain these electrons.
The oxidation number of i a group elements =+1. Oxidation number of a free element is zero. Oxidation number rule 1 the sum of oxidation numbers of all the atoms in a molecule is equal to zero.
Firstly, the oxidation numbers calculator draws the complete lewis diagram showing interacting atoms and valence electrons. Nacl → na⁺ + cl⁻ mgbr₂ → mg⁺² + 2br⁻ the algebraic sum of the oxidation numbers of all the atoms in a compound must be zero and the oxidation. The oxidation number of a monatomic ion equals the charge of the ion.
• group i metals (li + , na + , k + , etc) are almost always +1 in compounds. For example, the oxidation number of na + is +1; The oxidation number of an ion is equal to its charge.
Secondly, it is estimated which atom is gaining or donating the electron in the compound. The oxidation number of ii a group elements =+2. Oxygen in peroxides peroxides include hydrogen peroxide, h 2 o 2.
The calculation of the oxidation number is done by applying several oxidation number rules. Electrons draw towards more electronegative atoms and bond pairs. Rules to compute the oxidation numbers 1.
Chemists have developed a method to find which atoms have gained/lost electrons, especially since some reactions can seem very complicated. There are a set a rules that we use to determine oxidation number. This is an electrically neutral compound and so the sum of the oxidation states of the hydrogen and oxygen must be zero.
Here are the crucial rules to follow: The sum of oxidation numbers in a neutral compound is 0. Oxidation number of an atom in free state is equal to zero.
The sum of the oxidation number for all the atoms. To determine if electrons were gained or lost by an atom, we assign an oxidation number to each atom in a compound. This chemistry video tutorial provides a basic introduction on how to calculate oxidation numbers.
The usual oxidation number of hydrogen is +1. Group 1a elements (alkalai metals) always have an oxidation of +1. • the oxidation states of all the atoms in a neutral molecule add up to 0.
This video walks you through redox pairs using the mnemonic leo the lion says ger to help you understand oxidizing/reducing agents. Oxidation number of a monatomic ion is equal to charge of that ion. The common oxidation number of an element is equivalent to its group number from ia to iv a.
If you are given the h+ ion concentration then the ph is given by the negative logirthm of the hydrogen ion concentration. Best method is ion chromatography since it does the separation and measures the concentration of both species.

Chem 20 Molar Concentration Of Ions - Youtube
Mass percent composition (also called mass percent or percent composition) is the easiest way to express the concentration of a solution because no unit conversions are required.

How to find concentration of ions in a solution. If you know the ph, you can solve for the hydronium ion concentration and conversely, you can solve for ph if you know the concentration of hydronium ions. Here's how that would look mathematically: This video contains plenty of examples and practic.
Thus, you have 0.0037922 grams of n o x 3. Of sample solution in order to find the concentration of a certain ion in the sample solution. Multiplying this by the molar mass of iron gives you 0.00170775 grams of f e.
\text concentration in ppm = \frac \text {amount of solute} \text {amount of whole solution} × 10^6 concentration in ppm = amount of whole solutionamount of solute ×106 You got it priya thakur 1 y related the ph of a solution changes from 3 to 6. Ph = − log [h3o+] the ph of a solution is equal to the negative logarithm of the hydronium ion (h3o+) concentration.
Ion concentrations in solution when an ionic compound dissolves it breaks up into its ions. We know that concentration is typically expressed with molarity, which is moles per liter. N compoundn ion as you can see, the mole ratio between the original compound and an ion it forms will determine the concentration of the respective ion in solution.
Then you can use the following formula to find the concentration: Record these concentrations and ratio in table c. At 25 °c, we can correlate whether a solution is acidic, basic, or neutral based off of the measured ph of the solutions:
(don’t try to memorize this or even fully understand it yet. However, i am unsure if the process i used was correct. Molarity is defined as the number of moles of solute dissolved per liter of solution (mol/l = m).
This chemistry video tutorial explains how to calculate the ion concentration in solutions from molarity. A 1 m solution is one in which exactly 1 mole of solute is dissolved in a total solution volume of exactly 1 l. Simply use a scale to measure the mass of the solute and the final solution and express the ratio as a percentage.
N compoundc compound c ion=c compound. To get the concentration at the time when the lead iodide started dissolving, i took my answer from the first part of the earlier calculation ( 1.5 × 10 − 13 / ( 0.10 × 0.032 / 0.082)), for a final carbonate ion concentration of 3.8 × 10 − 12. The precipitation reaction involves 75.0 ml of.0750 m solution of potassium phosphate and 75.0 ml of.0750 m iron (ii) acetate.
This is also referred to as molarity, which is the most common method of expressing the concentration of a solute in a solution. But how do we know how many moles of solute are present in solution when an ionic solid dissolves in. C compound= vn compound⇒v= c compoundn compound c ion= vn ion=n ion.
Other tests include acidification to form co2 gas or using test strips. I'm very confused as to how to find the concentration of the remaining solutions, particularly because i am not sure if i am supposed to regard the coefficients that are in the balanced equation (for example, when i balanced the. The concentration of iron is c = f e w a t e r x + f e ( n o x 3) x 2 = 0.00170775 0.0055 + 2000 = 8.538726518 × 10 − 7 thus, f e has a p p m of 0.85 share improve this answer edited sep 29, 2018 at 21:16 a.k.
It is known that they can host. That's because it's 14 times bigger and about 100,000 times brighter than.

Video: We Watched A Red Supergiant Star's 'Dying Breath' Before It Exploded As A Supernova Say Scientists
Apr 13, 2018 see explanation explanation:

Red giant supernova. Betelgeuse is far away, yet it's one of the brightest stars in earth's sky. Massive red supergiant star betelgeuse is at the end of its life span, at least on cosmic timescales, but the gargantuan fireball is going out kicking and screaming. A red supergiant star transitions into a type ii supernova in this animation.
Both supernovas and red giants are dying stars. Facebook twitter linkedin comment mail Supernova in 1.5 million years.
Their core has used all or almost all their hydrogen and now they fuse heavier elements. After a supernova, a binary star may be composed of one red giant and one neutron star. Keck observatory/adam makarenko | mash mix by space.com's [steve.
Supernovas happen when massive stars die, or run out of fuel and collapse in on themselves, no longer to keep the forces of gravity and nuclear reactions in balance. A star maintains its stability through a fine balance between its. A recent study that was just published in the astrophysical journal shows how a select group of scientists witnessed firsthand a distant red supergiant star exploded into a.
The red giant can be torn apart by. A red giant forms after a star has run out of hydrogen fuel for nuclear fusion, and has begun the process of dying. Neutron star and red giant binary destruction.
Red giant betelgeuse might go supernova, and we better watch out for it. Staff reporter jan 06, 2020 01:15 am est. Both supernovas and red giants.
January 9, 2022 by brian wang. But finally, astronomers managed to observe a red giant star just as it went supernova, as exploding stars are called.using a telescope in hawaii, a team of scientists. Once stars that are 5 times or more massive than our sun reach the red giant phase, their core temperature increases as carbon atoms are formed from the fusion of helium.
Red giants are giant stars that are not located on the main sequence. When a red supergiant has burnt out most of its fuel it resulst in a gravitational collaps, lowering its volume making the star smaller. Medium sized stars become red giants, and very large stars become supernovas.
An artist's rendition of a red supergiant star transitioning into a type ii supernova, emitting a violent eruption of radiation and gas on its dying breath before collapsing and.
So in this way answer is (square root of 90 by long division method)/10. S q r t 0.9 =?

Evaluate √0.9 Correct Up To Two Places Of Decimal.
√0.9 = √9 ⋅ 0.1 = 3√0.1 or, if you want a more exact answer, you can use a calculator to get an approximate square root.

Square root of 0.9. Tp find square root of 90 use long division method. Related questions square root of 90 to nine decimals places? 0.9 = 9/10 =√(9/10) = 3/√10 = (3*√10) / (√10 * √10) = 3√10 / 10 = (3 * 3.16) / 10 = 9.48 / 10 = 0.948 ( approx)
Cube root of 0.9 = 0.96548938 use wizard instead? Cube of a number is its third power, while inverse is a multiplicative inverse or reciprocal of a number. S q r t f r a c 9 ∗ 10 10 ∗ 10.
To find the square root of 2, push: Now we have to multiply a number. Let , which is essentially the number of significant digits in the nth [/su] estimate.
Put a bar on each pair. The square root of 0. Use the square root calculator below to find the square root of any imaginary or real number.
A square root of a number ‘x’ is a number ‘y’ such that ‘square of y’ = ‘x’ and a cube root of a number ‘x’ is a number ‘y’ such that ‘cube of y’ = ‘x’. It is also expressed as. Inputs for the radicand x can be positive or negative real numbers.
The square root of 2000 is 44.713595544.72135955 to eight decimal places. To find the square root of 9 by the long division method, we need to follow the steps given below. Square root of 0.9 explain briefly.
This happens to be a very good initial guess. Make a pair of digits of the given number starting with a digit at the unit's place. To take the square root of a number, press [2nd] (the secondary function key) and then [√ ] (the radical symbol key which is used to take the square root of a number) and then the number that you want to find the square root of and then the [enter] key.example:
Use this calculator to find the principal square root and roots of real numbers. √0.9 ≈ 0.94868329805 answer link And square root of 100 is 10.
Take the square root on both sides: But i guess you can simplify it instead. The answer will show you the complex or imaginary solutions for square roots of negative real numbers.
Promoted the penny hoarder kyle taylor As 0.9 can be written as 9/10. S q r t f r a c 90 100.
The square root is an irrational number, so you won't be able to get an exact answer for that. (i) 0.9 class 8 rd sharma leave an answer 1 answer added an answer on december 29, 2021 at 11:45 am The square root of 0.9 is 0.3.
The answer will also tell you if you entered a perfect square. See also in this web page a square root table from 1 to 100 as well as the babylonian method or hero's method. Answered jul 28, 2020 by rani01 (52.4k points) selected jul 29, 2020.
Simplification questions & answers for bank exams : The correct statement is that the square root of 0.9 is approxiamately 0.9 0.9 ≈ 0.9 share answered may 15, 2016 at 15:07 simply beautiful art 72.2k 11 112 252 add a comment Now multiply and divide this number by 10 so the number becomes 90/100 now find square root 90 and 100 individually.
Find square root of 0.9 correct to three places of decimal find the square root of each of the following correct to three places of decimal. 9.486832981 what is the square root of 226? Long division method,maths,square root.square root of 0.9 using long division method
Square root of 0.9 | √0.9 or what is the square root of 0.9? The square root of 0.9 is: So, right off the bat, the initial guess is good to 2.86 decimal places.
Square root of 0.9 is equal to [2nd] [√ ] 2 [enter] this will give you the answer of: Therefore 0.95 ^ 2 = 0.9025 nearly equal to 0.9 approximate value is 0.95 easy way of finding 95 * 95 is • • ( 5 x 5) = ••25 •• is found by multiplying 9 and its next number 10 , i.e 90 so 95 * 95 = 90 25 = 9025 douglas porter b sc in mathematics, the open university (graduated 2011) 1 y share
Multiply by 10 on numerator and denominator side. Here is the answer to questions like: The square root of 0.9 is obviously somewhere between 0.9 and 1, so a good place to start is the average of these two.
S q r t f r a c 9 10. Share it on facebook twitter email.
Billy graham explains more in this 1983 mes. Let's ask & get answers log in sign up physics chemistry math biology science economy business studies accountancy sociology social science political science geography computer science history india languages elements.

Cot As A Function Of Walking Speed For All Trials. | Download Scientific Diagram
For most people, the 3 main payment dates are:

Cot of 27. 27 may 2022 you may be able to get a payment to help with the cost of living if you’re in receipt of certain benefits or tax credits. The coat of arms of south africa is the main heraldic insignia of south africa.the present coat of arms was introduced on freedom day, 27 april 2000, and was designed by mr iaan bekker. In most cases, breast cancer is responsible for the increase in ca 27.29 levels.
On october 27, 1939, nylon officially unveiled. Commodity futures trading commission | cftc The total cost of living payment is $350 which will be split into 3 monthly payments starting 1 august.
To follow christ truly, we must consider the cost. For the cotangent of 5.27 we use the abbreviation cot for the trigonometric function and write it as cot 5.27. Ca 27.29 is an antigen that is released into the blood by specific cancer cells.
Cot 81° = cot 9°. The cost of new dwellings rose 5.6% fuelled by shortages of building supplies, higher shipping costs and continued high levels of construction activity. Measuring the amount of ca 27.29 in blood primarily helps monitor cancer.
Solution for cot 27° start your trial now! If you have been looking for what is cot 5.27, or if you have been wondering about cot 5.27 radians in degrees, then you are right here, too. A+ cot²a 1 see answer advertisement
Welcome to cot 5.27, our post aboutthe cotangent of 5.27. Divyamr344 divyamr344 22.11.2019 math secondary school answered cot 9 degree cot 27 degree cot 45 degree cot. If cot a+ cot a 1 = 2, find the value of cot?
From october, two further levies will also come into force: It stimulates body’s defence system and acts as a tumour marker, providing information about the cancer. The planet is made up of 72% saltwater and 1% freshwater, leaving 27% of the world as land.
You do not need to apply, if you’re eligible, you’ll be paid. The motto is written in the khoisan language of the ǀxam people and translates literally to diverse people unite. If cot a+cot a1= 2, find the value of cot?
It replaced the earlier national arms, which had been in use since 1910. In this post you can find the cot 5.27 value, along with identities. Then he states a third cost of discipleship (14:33).
The federal government also offered fewer. A+cot²a get the answers you need, now! Itsanuragsingh itsanuragsingh 11.07.2020 math secondary school answered 27.
Cot 9 degree cot 27 degree cot 45 degree cot 63 degree cot 81 degree equals 1 get the answers you need, now! Find the exact value cot ( (27pi)/2) cot ( 27π 2) cot ( 27 π 2) subtract full rotations of 2π 2 π until the angle is greater than or equal to 0 0 and less than 2π 2 π. Marvel’s deadpool issue #27 holds the guinness world record for the most comic book characters on one cover.
The number 27 is often referred to as the trinity of trinities as 3x3x3=27, 3×3=9, and 3×9=27. When we choose to follow christ, it is a matter of the heart that requires a total shift in thoughts and actions. Cot( 3π 2) cot ( 3 π 2) apply the reference angle by finding the angle with equivalent trig values in the first quadrant.
1 the taste of halogenated organic compounds mainly depends on the halogen atoms involved. The three general requirements for a.
Secret Of Liquorice's Unique Smell Unravelled | Research | Chemistry World
They found that hydrocarbons, alcohols, ketones and aldehydes were present in large quantities in.
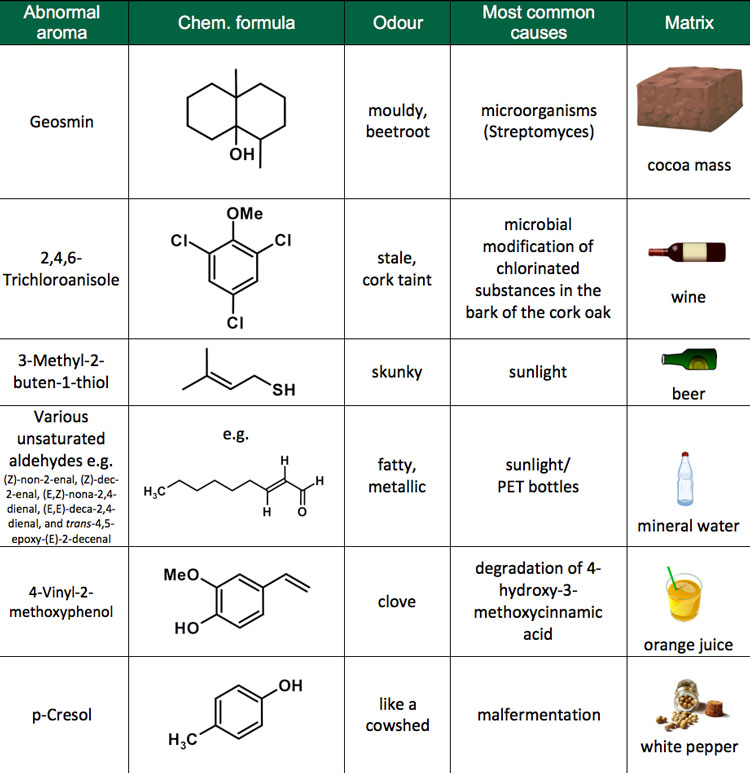
Why do aromatic compounds smell. The word aromatic originated from the greek aroma meaning aroma. Paper contains, amongst other chemicals, cellulose, and smaller. It turns out, the stinky culprits in both substances belong to a family of prenylated volatile sulfur compounds.
What is the reason behind its. But why do they actually smell? When food is consumed, since most of the aromatic compounds are derivatives of benzene and benzene gives distinct odor so, which can be increased up to 1000 most commonly associated.
Why do aromatic compounds smellole henriksen walnut scrub sephora. Aromatics, so called because of their distinctive perfumed smell, are substances derived from crude oil and, in small quantities, from coal. Study showed that halogenation of aliphatic series imparted.
Esters smell partly because they exhibit weak intermolecular forces. These compounds are called aromatic compounds due to their special type of smell. Many aromatic compounds are however, sweet/pleasant smelling.
They have a vapour pressure at ambient settings, which means that enough molecules are in the. Why do aromatic compounds smell? But attainment of slight polarity only due to the sp2 hybridised carbon which.
We all know the very famous esterification reaction, where a carboxylic acid and an alcohol react to give an ester and water. In other words, why does cannabis smell so much like skunk spray? This allows ester molecules to enter the gas phase and reach your nose.
New york knicks best moments; They are known as aromatic due to their pleasant smell. Aromatic compounds are broadly divided into two categories:
The researchers compiled an exhaustive list of the volatile compounds present; Generally, it is the chemical breakdown of compounds within paper that leads to the production of ‘old book smell’.
